Diclofenac Potassium Prescribing Information
- Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use (see
WARNINGS). - Diclofenac potassium tablets are contraindicated in the setting of coronary artery bypass graft (CABG) surgery (see
CONTRAINDICATIONS,
WARNINGS).
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events (see
WARNINGS).
Carefully consider the potential benefits and risks of diclofenac potassium tablets and other treatment options before deciding to use diclofenac potassium tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see
WARNINGS: Gastrointestinal Bleeding, Ulceration, and Perforation).
Diclofenac potassium tablets are indicated:
- for treatment of primary dysmenorrhea
- for relief of mild to moderate pain
- for relief of the signs and symptoms of osteoarthritis
- for relief of the signs and symptoms of rheumatoid arthritis
Carefully consider the potential benefits and risks of diclofenac potassium tablets and other treatment options before deciding to use diclofenac potassium tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see
WARNINGS: Gastrointestinal Bleeding, Ulceration, and Perforation).
After observing the response to initial therapy with diclofenac potassium tablets, the dose and frequency should be adjusted to suit an individual patient’s needs.
For treatment of pain or primary dysmenorrhea the recommended dosage is 50 mg three times a day. With experience, physicians may find that in some patients an initial dose of 100 mg of diclofenac potassium tablets, followed by 50 mg doses, will provide better relief.
For the relief of osteoarthritis the recommended dosage is 100 to 150 mg/day in divided doses, 50 mg twice a day or three times a day.
For the relief of rheumatoid arthritis the recommended dosage is 150 to 200 mg/day in divided doses, 50 mg three times a day or four times a day. Different formulations of diclofenac [VOLTAREN® (diclofenac sodium enteric-coated tablets; Voltaren®-XR (diclofenac sodium extended-release tablets); diclofenac potassium immediate-release tablets)] are not necessarily bioequivalent even if the milligram strength is the same.
Diclofenac potassium tablets are contraindicated in the following patients:
- Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to diclofenac or any components of the drug product (see
WARNINGS: Anaphylactic Reactions, Serious Skin Reactions). - History of asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients (see
WARNINGS: Anaphylactic Reactions, Exacerbation of Asthma Related to Aspirin Sensitivity). - In the setting of coronary artery bypass graft (CABG) surgery (see
WARNINGS: Cardiovascular Thrombotic Events).
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Cardiovascular Thrombotic Events (see
WARNINGS) - GI Bleeding, Ulceration and Perforation (see
WARNINGS) - Hepatotoxicity (see
WARNINGS) - Hypertension (see
WARNINGS) - Heart Failure and Edema (see
WARNINGS) - Renal Toxicity and Hyperkalemia (see
WARNINGS) - Anaphylactic Reactions (see
WARNINGS) - Serious Skin Reactions (see
WARNINGS) - Hematologic Toxicity (see
WARNINGS)
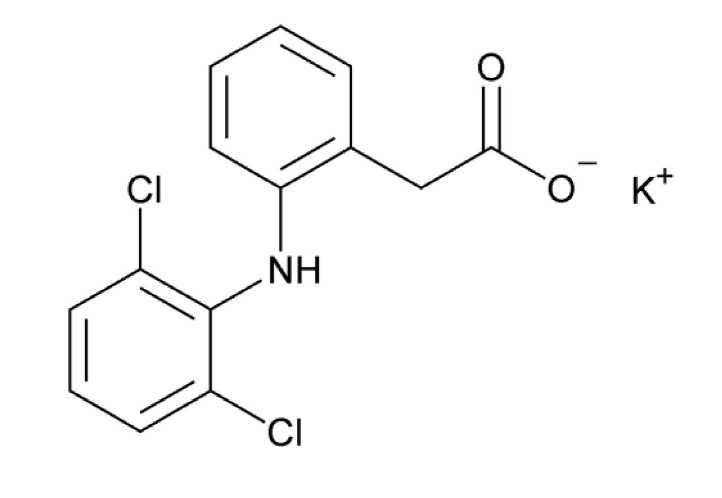
Diclofenac potassium tablets, USP are a benzeneacetic acid derivative. Diclofenac potassium tablets are available for oral administration. Diclofenac potassium, USP is a white to off-white or slightly yellowish crystalline powder, slightly hygroscopic and is sparingly soluble in water, Freely soluble in methanol; soluble in alcohol, slightly soluble in acetone. The chemical name is Potassium [ o-(2,6-dichloroanilino)phenyl] acetate. The molecular weight is 334.24. Its molecular formula is C 14H 10Cl 2KNO 2, and it has the following structural formula:
The inactive ingredients in diclofenac potassium tablets include: Lactose Anhydrous, Microcrystalline Cellulose, NF, Colloidal Silicon Dioxide, NF, Croscarmellose Sodium, NF, Magnesium Stearate, NF, Titanium Dioxide, USP, Polydextrose, NF, Hypromellose, USP 2910 (6 mPas), Hypromellose, USP 2910 (3 mPas), Hypromellose, USP 2910 (50 mPas), Triacetin, USP and Polyethylene Glycol, NF 8000.