Differin
(Adapalene)Differin Prescribing Information
DIFFERIN Gel is indicated for the topical treatment of acne vulgaris in patients 12 years of age and older.
Wash affected areas gently with a non-medicated soap. Apply a thin film of DIFFERIN Gel to the entire face and any other affected areas of the skin once daily in the evening. Avoid application to the areas of skin around eyes, lips, and mucous membranes. A mild transitory sensation of warmth or slight stinging may occur shortly after the application of DIFFERIN Gel.
Instruct patients to minimize sun exposure and to use moisturizers for relief of dry skin or irritation.
If therapeutic results are not noticed after 12 weeks of treatment, therapy should be re-evaluated.
For topical use only. Not for ophthalmic, oral or intravaginal use.
Each gram of DIFFERIN Gel, 0.3% contains 3 mg adapalene in an off-white aqueous gel.
DIFFERIN Gel is contraindicated in patients who have known hypersensitivity to adapalene or any excipient of DIFFERIN Gel [
Adverse reactions including anaphylaxis angioedema, face edema, eyelid edema, lip swelling, and pruritus that sometimes required medical treatment have been reported during postmarketing use of adapalene. Advise a patient to stop using DIFFERIN Gel and seek medical attention if experiencing allergic or anaphylactoid/anaphylactic reactions during treatment.
DIFFERIN (adapalene) Gel contains adapalene 0.3% (3 mg/g) in a topical aqueous gel for use in the treatment of acne vulgaris, consisting of carbomer 940, edetate disodium, methylparaben, poloxamer 124, propylene glycol, purified water, and sodium hydroxide. May contain hydrochloric acid for pH adjustment.
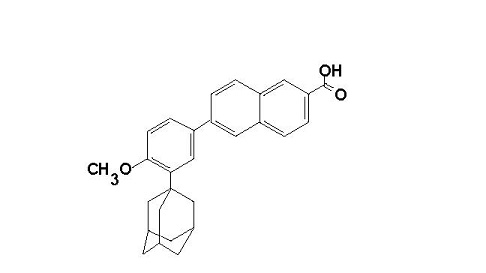
The chemical name of adapalene is 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid. It is a white to off-white powder, which is soluble in tetrahydrofuran, very slightly soluble in ethanol, and practically insoluble in water. The molecular formula is C28H28O3 and molecular weight is 412.53. Adapalene is represented by the following structural formula.
The safety and efficacy of once daily use of DIFFERIN Gel for treatment of acne vulgaris were assessed in one 12 week, multi-center, controlled, clinical trial, conducted in a total of 653 subjects 12 to 52 years of age with acne vulgaris of mild to moderate severity. All female subjects of child-bearing potential enrolled in the trial were required to have a negative urine pregnancy test at the beginning of the trial and were required to practice a highly effective method of contraception during the trial. Female subjects who were pregnant, nursing or planning to become pregnant were excluded from the trial.
Subjects enrolled in the trial were Caucasian (72%), Hispanic (12%), African-American (10%), Asian (3%), and other (2%). An equal number of males (49.5%) and females (50.5%) enrolled. Success was defined as “Clear” or “Almost Clear” in the Investigator’s Global Assessment (IGA). The success rate, mean reduction, and percent reduction in acne lesion counts from Baseline after 12 weeks of treatment are presented in the following table:
DIFFERIN (adapalene) Gel, 0.3% | Adapalene Gel, 0.1% | Vehicle Gel | |
N = 258 | N = 261 | N = 134 | |
| IGA Success Rate | 53 (21%) | 41 (16%) | 12 (9%) |
| Inflammatory Lesions Mean Baseline Count Mean Absolute (%) Reduction | 27.7 14.4 (51.6%) | 28.1 13.9 (49.7%) | 27.2 11.2 (40.7%) |
| Non-Inflammatory Lesions Mean Baseline Count Mean Absolute (%) Reduction | 39.4 16.3 (39.7%) | 41.0 15.2 (35.2%) | 40.0 10.3 (27.2%) |
| Total Lesions Mean Baseline Count Mean Absolute (%) Reduction | 67.1 30.6 (45.3%) | 69.1 29.0 (41.8%) | 67.2 21.4 (33.7%) |