Dihydroergotamine Mesylate Nasal Prescribing Information
Dihydroergotamine mesylate nasal spray is indicated for the acute treatment of migraine headaches with or without aura.
Dihydroergotamine mesylate nasal spray is not intended for the prophylactic therapy of migraine or for the management of hemiplegic or basilar migraine.
In clinical trials, dihydroergotamine mesylate nasal spray has been effective for the acute treatment of migraine headaches with or without aura. One spray (0.5 mg) of dihydroergotamine mesylate nasal spray should be administered in each nostril. Fifteen minutes later, an additional one spray (0.5 mg) of dihydroergotamine mesylate nasal spray should be administered in each nostril, for a total dosage of four sprays (2 mg) of dihydroergotamine mesylate nasal spray. Studies have shown no additional benefit from acute doses greater than 2 mg for a single migraine administration. The safety of doses greater than 3 mg in a 24 hour period and 4 mg in a 7 day period has not been established.
Dihydroergotamine mesylate nasal spray, should not be used for chronic daily administration.
Prior to administration, the pump must be primed (i.e., squeeze 4 times) before use (see administration instructions). Once the nasal spray applicator has been prepared, it should be discarded (with any remaining drug in opened vial) after 8 hours.
In addition to those conditions mentioned above, dihydroergotamine mesylate nasal spray is also contraindicated in patients with known peripheral arterial disease, sepsis, following vascular surgery, and severely impaired hepatic or renal function.
Dihydroergotamine mesylate nasal spray is contraindicated in patients who have previously shown hypersensitivity to ergot alkaloids.
Dihydroergotamine mesylate should not be used with peripheral and central vasoconstrictors because the combination may result in additive or synergistic elevation of blood pressure.
Serious cardiac events, including some that have been fatal, have occurred following use of the parenteral form of dihydroergotamine mesylate (D.H.E. 45®Injection), but are extremely rare. Events reported have included coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, and ventricular fibrillation
Fibrotic complications have been reported in association with long term use of injectable dihydroergotamine mesylate
Of the 1,796 patients and subjects treated with dihydroergotamine mesylate nasal spray doses 2 mg or less in U.S. and foreign clinical studies, 26 (1.4%) discontinued because of adverse events.
The adverse events associated with discontinuation were, in decreasing order of frequency: rhinitis 13, dizziness 2, facial edema 2, and one each due to cold sweats, accidental trauma, depression, elective surgery, somnolence, allergy, vomiting, hypotension, and paraesthesia.
The most commonly reported adverse events associated with the use of dihydroergotamine mesylate nasal spray during placebo-controlled, double-blind studies for the treatment of migraine headache and not reported at an equal incidence by placebo-treated patients were rhinitis, altered sense of taste, application site reactions, dizziness, nausea, and vomiting. The events cited reflect experience gained under closely monitored conditions of clinical trials in a highly selected patient population. In actual clinical practice or in other clinical trials, these frequency estimates may not apply, as the conditions of use, reporting behavior, and the kinds of patients treated may differ.
Dihydroergotamine mesylate nasal spray was generally well tolerated. In most instances these events were transient and self-limited and did not result in patient discontinuation from a study. The following table summarizes the incidence rates of adverse events reported by at least 1% of patients who received dihydroergotamine mesylate nasal spray for the treatment of migraine headaches during placebo-controlled, double-blind clinical studies and were more frequent than in those patients receiving placebo.
Table 3: Adverse Reaction Reported by at least 1% of the Dihydroergotamine Mesylate Nasal Spray Treated Patients and Occurred More Frequently than in the Placebo-Group in the Migraine Placebo-Controlled Trials | ||
Dihydroergotamine mesylate nasal spray N=597 | Placebo N=631 | |
Respiratory System | ||
| Rhinitis Pharyngitis Sinusitis | 26% 3% 1% | 7% 1% 1% |
Gastrointestinal System | ||
| Nausea Vomiting Diarrhea | 10% 4% 2% | 4% 1% <1% |
Special Senses, Other | ||
| Altered Sense of Taste | 8% | 1% |
Application Site | ||
| Application Site Reaction | 6% | 2% |
Central and Peripheral Nervous System | ||
| Dizziness Somnolence Paraesthesia | 4% 3% 2% | 2% 2% 2% |
Body as a Whole, General | ||
| Hot Flashes Fatigue Asthenia | 1% 1% 1% | <1% 1% 0% |
Autonomic Nervous System | ||
| Mouth Dry | 1% | 1% |
Musculoskeletal System | ||
| Stiffness | 1% | <1% |
Other Adverse Events During Clinical Trials
In the paragraphs that follow, the frequencies of less commonly reported adverse clinical events are presented. Because the reports include events observed in open and uncontrolled studies, the role of dihydroergotamine mesylate nasal spray in their causation cannot be reliably determined. Furthermore, variability associated with adverse event reporting, the terminology used to describe adverse events, etc., limit the value of the quantitative frequency estimates provided. Event frequencies are calculated as the number of patients who used dihydroergotamine mesylate nasal spray in placebo-controlled trials and reported an event divided by the total number of patients (n=1796) exposed to dihydroergotamine mesylate nasal spray. All reported events are included except those already listed in the previous table, those too general to be informative, and those not reasonably associated with the use of the drug. Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1,000 patients; and rare adverse events are those occurring in fewer than 1/1,000 patients.
Rare: anxiety, anorexia, depression.
Voluntary reports of adverse events temporally associated with dihydroergotamine products used in the management of migraine that have been received since the introduction of the injectable formulation are included in this section save for those already listed above. Because of their source (open and uncontrolled clinical use), whether or not events reported in association with the use of dihydroergotamine are causally related to it cannot be determined. There have been reports of pleural and retroperitoneal fibrosis in patients following prolonged daily use of injectable dihydroergotamine mesylate. Dihydroergotamine mesylate nasal spray is not recommended for prolonged daily use.
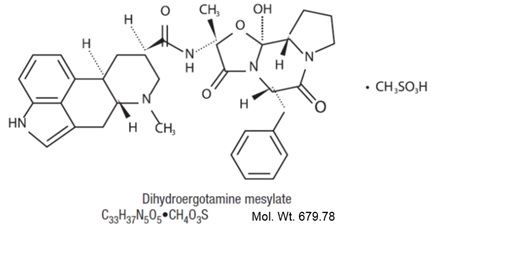
Dihydroergotamine mesylate is ergotamine hydrogenated in the 9,10 position as the mesylate salt. Dihydroergotamine mesylate is known chemically as ergotaman-3', 6', 18-trione, 9,10-dihydro-12'-hydroxy-2'-methyl-5'- (phenylmethyl)-, (5'α)-, monomethane-sulfonate. Its molecular weight is 679.78 and its empirical formula is C33H37N5O5•CH4O3S.
The chemical structure is:
Dihydroergotamine mesylate nasal spray is provided for intranasal administration as a clear, colorless to faintly yellow solution in an amber glass vial containing:
dihydroergotamine mesylate……………4 mg
caffeine, anhydrous……………………. 10 mg
dextrose, anhydrous…………………… 50 mg
carbon dioxide………………………….qs
purified water ………………………….qs 1 mL
Each milliliter contains
Dihydroergotamine mesylate……4 mg
(equivalent to 3.43 mg dihydroergotamine)