Diltiazem Hydrochloride
Diltiazem Hydrochloride Prescribing Information
Diltiazem Hydrochloride Injection is indicated for the following:
The initial dose of diltiazem hydrochloride injection should be 0.25 mg/kg actual body weight as a bolus administered over 2 minutes (20 mg is a reasonable dose for the average patient). If response is inadequate, a second dose may be administered after 15 minutes. The second bolus dose of diltiazem hydrochloride injection should be 0.35 mg/kg actual body weight administered over 2 minutes (25 mg is a reasonable dose for the average patient). Subsequent intravenous bolus doses should be individualized for each patient. Patients with low body weights should be dosed on a mg/kg basis. Some patients may respond to an initial dose of 0.15 mg/kg, although duration of action may be shorter. Experience with this dose is limited.
Diltiazem hydrochloride injection is contraindicated in:
- Patients with sick sinus syndrome except in the presence of a functioning ventricular pacemaker.
- Patients with second- or third-degree AV block except in the presence of a functioning ventricular pacemaker.
- Patients with severe hypotension or cardiogenic shock.
- Patients who have demonstrated hypersensitivity to the drug.
- Intravenous diltiazem and intravenous beta-blockers should not be administered together or in close proximity (within a few hours).
- Patients with atrial fibrillation or atrial flutter associated with an accessory bypass tract such as in WPW syndrome or short PR syndrome. As with other agents which slow AV nodal conduction and do not prolong the refractoriness of the accessory pathway (e.g., verapamil, digoxin), in rare instances patients in atrial fibrillation or atrial flutter associated with an accessory bypass tract may experience a potentially life-threatening increase in heart rate accompanied by hypotension when treated with diltiazem hydrochloride injection. As such, the initial use of diltiazem hydrochloride injection should be, if possible, in a setting where monitoring and resuscitation capabilities, including DC cardioversion/defibrillation, are present (see ). Once familiarity of the patient’s response is established, use in an office setting may be acceptable.OVERDOSAGE
Overdosage experience is limited. In the event of overdosage or an exaggerated response, appropriate supportive measures should be employed. The following measures may be considered:
BradycardiaAdminister atropine (0.6 to 1 mg). If there is no response to vagal blockade administer isoproterenol cautiously.
High-Degree AV BlockTreat as for bradycardia above. Fixed high-degree AV block should be treated with cardiac pacing.
Cardiac FailureAdminister inotropic agents (isoproterenol, dopamine, or dobutamine) and diuretics.
HypotensionVasopressors (e.g., dopamine or levarterenol bitartrate).
The effectiveness of intravenous calcium administration to reverse the pharmacological effects of diltiazem overdose has been inconsistent. In a few reported cases, overdose with calcium channel blockers associated with hypotension and bradycardia that was initially refractory to atropine became more responsive to atropine after the patients received intravenous calcium. In some cases intravenous calcium has been administered (1 g calcium chloride or 3 g calcium gluconate) over 5 minutes, and repeated every 10 to 20 minutes as necessary. Calcium gluconate has also been administered as a continuous infusion at a rate of 2 g per hour for 10 hours. Infusions of calcium for 24 hours or more may be required. Patients should be monitored for signs of hypercalcemia.
Actual treatment and dosage should depend on the severity of the clinical situation and the judgment and experience of the treating physician.
Diltiazem does not appear to be removed by peritoneal or hemodialysis. Limited data suggest that plasmapheresis or charcoal hemoperfusion may hasten diltiazem elimination following overdose.
The intravenous LD50s in mice and rats were 60 and 38 mg/kg, respectively. The toxic dose in man is not known.
- Patients with ventricular tachycardia. Administration of other calcium channel blockers to patients with wide complex tachycardia (QRS ≥0.12 seconds) has resulted in hemodynamic deterioration and ventricular fibrillation. It is important that an accurate pretreatment diagnosis distinguish wide complex QRS tachycardia of superventricular origin from that of ventricular origin prior to administration of diltiazem hydrochloride injection.
The following adverse reaction rates are based on the use of diltiazem hydrochloride injection in over 400 domestic clinical trial patients with atrial fibrillation/flutter or PSVT under double-blind or open-label conditions. Worldwide experience in over 1300 patients was similar.
Adverse events reported in controlled and uncontrolled clinical trials were generally mild and transient. Hypotension was the most commonly reported adverse event during clinical trials. Asymptomatic hypotension occurred in 4.3% of patients. Symptomatic hypotension occurred in 3.2% of patients. When treatment for hypotension was required, it generally consisted of administration of saline or placing the patient in the Trendelenburg position. Other events reported in at least 1% of the diltiazem-treated patients were injection site reactions (e.g., itching, burning) - 3.9%, vasodilation (flushing) - 1.7%, and arrhythmia (junctional rhythm or isorhythmic dissociation) - 1%.
In addition, the following events were reported infrequently (less than 1%):
As with all drugs, care should be exercised when treating patients with multiple medications. Diltiazem is both a substrate and an inhibitor of the cytochrome P-450 3A4 enzyme system. Other drugs that are specific substrates, inhibitors, or inducers of this enzyme system may have a significant impact on the efficacy and side effect profile of diltiazem. Patients taking other drugs that are substrates of CYP450 3A4, especially patients with renal and/or hepatic impairment, may require dosage adjustment when starting or stopping concomitantly administered diltiazem in order to maintain optimum therapeutic blood levels.
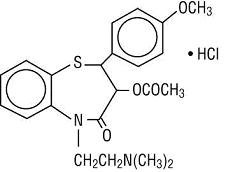
C22H26N2O4S• HCl MW: 450.98
Diltiazem hydrochloride is a white to off-white crystalline powder with a bitter taste. It is soluble in water, methanol, and chloroform.
Diltiazem hydrochloride injection is a clear, colorless, sterile, nonpyrogenic solution. It has a pH range of 3.7 to 4.1.
Diltiazem hydrochloride injection is for direct intravenous bolus injection and continuous intravenous infusion.
Each mL contains: 5 mg Diltiazem Hydrochloride, USP, 0.75 mg Citric Acid Anhydrous, USP, 0.65 mg Sodium Citrate Dihydrate USP, 50 mg Sorbitol NF, and Water for Injection, USP q.s. Sodium Hydroxide or Hydrochloric Acid is used to adjust pH.