Dipyridamole
Dipyridamole Prescribing Information
Dipyridamole tablets are indicated as an adjunct to coumarin anticoagulants in the prevention of postoperative thromboembolic complications of cardiac valve replacement.
The recommended dose is 75 mg to 100 mg four times daily as an adjunct to the usual warfarin therapy. Please note that aspirin is not to be administered concomitantly with coumarin anticoagulants.
Hypersensitivity to dipyridamole and any of the other components.
Adverse reactions at therapeutic doses are usually minimal and transient. On long-term use of dipyridamole tablets initial side effects usually disappear. The following reactions in Table 1 were reported in two heart valve replacement trials comparing dipyridamole tablets and warfarin therapy to either warfarin alone or warfarin and placebo:
| Adverse Reaction | Dipyridamole Tablets/ Warfarin | Placebo/ Warfarin |
|---|---|---|
| Number of patients | 147 | 170 |
| Dizziness | 13.6% | 8.2% |
| Abdominal distress | 6.1% | 3.5% |
| Headache | 2.3% | 0.0% |
| Rash | 2.3% | 1.1% |
Other reactions from uncontrolled studies include diarrhea, vomiting, flushing and pruritus. In addition, angina pectoris has been reported rarely and there have been rare reports of liver dysfunction. On those uncommon occasions when adverse reactions have been persistent or intolerable, they have ceased on withdrawal of the medication.
When dipyridamole tablets were administered concomitantly with warfarin, bleeding was no greater in frequency or severity than that observed when warfarin was administered alone. In rare cases, increased bleeding during or after surgery has been observed.
In post-marketing reporting experience, there have been rare reports of hypersensitivity reactions (such as rash, urticaria, severe bronchospasm, and angioedema), larynx edema, fatigue, malaise, myalgia, arthritis, nausea, dyspepsia, paresthesia, hepatitis, thrombocytopenia, alopecia, cholelithiasis, hypotension, palpitation, and tachycardia.
No pharmacokinetic drug-drug interaction studies were conducted with dipyridamole tablets. The following information was obtained from the literature.
Dipyridamole is a platelet inhibitor chemically described as 2,2',2",2'''-[(4,8-Dipiperidinopyrimido[5,4-
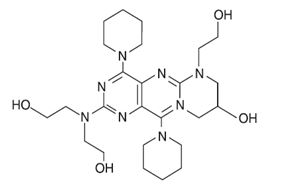
Dipyridamole, USP is intensely yellow crystalline powder or needles. It is practically insoluble in water, sparingly soluble in ethyl alcohol, very slightly soluble in acetone and ethyl acetate.
Each tablet, for oral administration, contains 25 mg, 50 mg or 75 mg dipyridamole, USP. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, povidone, pregelatinized starch, sodium starch glycolate, Type A, talc, and titanium dioxide.