Divalproex Sodium Prescribing Information
Divalproex sodium delayed-release tablets are intended for oral administration. Divalproex sodium delayed-release tablets should be swallowed whole and should not be crushed or chewed.
Patients should be informed to take divalproex sodium delayed-release tablets every day as prescribed. If a dose is missed it should be taken as soon as possible, unless it is almost time for the next dose. If a dose is skipped, the patient should not double the next dose.
Divalproex sodium delayed-release tablets are supplied as:
125 mg white to off white-colored tablets
250 mg pale brown-colored tablets
500 mg blue-colored tablets
- Divalproex sodium delayed-release tablets should not be administered to patients with hepatic disease or significant hepatic dysfunction [see Warnings and Precautions ()].5.1 HepatotoxicityGeneral Information on Hepatotoxicity
Hepatic failure resulting in fatalities has occurred in patients receiving valproate. These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months of valproate therapy. However, healthcare providers should not rely totally on serum biochemistry since these tests may not be abnormal in all instances, but should also consider the results of careful interim medical history and physical examination.
Caution should be observed when administering valproate products to patients with a prior history of hepatic disease. Patients on multiple anticonvulsants, children, those with congenital metabolic disorders, those with severe seizure disorders accompanied by mental retardation, and those with organic brain disease may be at particular risk. See below, “Patients with Known or Suspected Mitochondrial Disease.”
Experience has indicated that children under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those with the aforementioned conditions. When divalproex sodium is used in this patient group, it should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. In progressively older patient groups experience in epilepsy has indicated that the incidence of fatal hepatotoxicity decreases considerably.
Patients with Known or Suspected Mitochondrial DiseaseDivalproex sodium is contraindicated in patients known to have mitochondrial disorders caused by POLG mutations and children under two years of age who are clinically suspected of having a mitochondrial disorder
[see Contraindications (4)]. Valproate-induced acute liver failure and liver-related deaths have been reported in patients with hereditary neurometabolic syndromes caused by mutations in the gene for mitochondrial DNA polymerase γ (POLG) (e.g., Alpers-Huttenlocher Syndrome) at a higher rate than those without these syndromes. Most of the reported cases of liver failure in patients with these syndromes have been identified in children and adolescents.POLG-related disorders should be suspected in patients with a family history or suggestive symptoms of a POLG-related disorder, including but not limited to unexplained encephalopathy, refractory epilepsy (focal, myoclonic), status epilepticus at presentation, developmental delays, psychomotor regression, axonal sensorimotor neuropathy, myopathy cerebellar ataxia, ophthalmoplegia, or complicated migraine with occipital aura. POLG mutation testing should be performed in accordance with current clinical practice for the diagnostic evaluation of such disorders. The A467T and W748S mutations are present in approximately 2/3 of patients with autosomal recessive POLG-related disorders.
In patients over two years of age who are clinically suspected of having a hereditary mitochondrial disease, divalproex sodium should only be used after other anticonvulsants have failed. This older group of patients should be closely monitored during treatment with divalproex sodium for the development of acute liver injury with regular clinical assessments and serum liver test monitoring.
The drug should be discontinued immediately in the presence of significant hepatic dysfunction, suspected or apparent. In some cases, hepatic dysfunction has progressed in spite of discontinuation of drug
[see Boxed Warning and Contraindications (4)]. - Divalproex sodium delayed-release tablets are contraindicated in patients known to have mitochondrial disorders caused by mutations in mitochondrial DNA polymerase γ (POLG; e.g., Alpers-Huttenlocher Syndrome) and children under two years of age who are suspected of having a POLG-related disorder [see Warnings and Precautions (5.1)].
- Divalproex sodium delayed-release tablets are contraindicated in patients with known hypersensitivity to the drug [see Warnings and Precautions (5.12)].
- Divalproex sodium delayed-release tablets are contraindicated in patients with known urea cycle disorders [see Warnings and Precautions (5.6)].
- For use in prophylaxis of migraine headaches: Divalproex sodium delayed-release tablets are contraindicated in women who are pregnant and in women of childbearing potential who are not using effective contraception[see Warnings and Precautions (5.2, 5.3, 5.4) and Use in Specific Populations (8.1)].
The following serious adverse reactions are described below and elsewhere in the labeling:
- Hepatic failure [see Warnings and Precautions (5.1)]
- Birth defects [see Warnings and Precautions (5.2)]
- Decreased IQ following in utero exposure [see Warnings and Precautions (5.3)]
- Pancreatitis[see Warnings and Precautions (5.5)]
- Hyperammonemic encephalopathy [see Warnings and Precautions (5.6, 5.9, 5.10)]
- Suicidal behavior and ideation [see Warnings and Precautions (5.7)]
- Bleeding and other hematopoietic disorders [see Warnings and Precautions (5.8)]
- Hypothermia [see Warnings and Precautions (5.11)]
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan hypersensitivity reactions [see Warnings and Precautions (5.12)]
- Somnolence in the elderly [see Warnings and Precautions (5.14)]
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Divalproex Sodium, USP is a stable co-ordination compound comprised of sodium valproate and valproic acid in a 1:1 molar relationship and formed during the partial neutralization of valproic acid with 0.5 equivalent of sodium hydroxide. Chemically it is designated as sodium hydrogen bis(2-propylpentanoate). Divalproex Sodium, USP has the following structure:
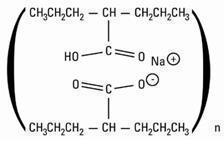
Divalproex Sodium, USP occurs as a white powder with a characteristic odor.
Divalproex sodium delayed-release tablets, USP are for oral administration. Divalproex sodium delayed-release tablets, USP are supplied in three dosage strengths containing divalproex sodium, USP equivalent to 125 mg, 250 mg, or 500 mg of valproic acid.
Inactive Ingredients: hypromellose, methacrylic acid co-polymer, microcrystalline cellulose, polyethylene glycol 6000, povidone, pregelatinized starch (contains corn starch), silicon dioxide, simethicone emulsion, sodium hydroxide, talc, and triethyl citrate.
In addition, individual tablets contain:
250 mg tablets: Ferric oxide.
500 mg tablets: FD&C Blue No. 1.
The components of imprinting ink are ammonium hydroxide, iron oxide black, isopropyl alcohol, n-butyl alcohol, propylene glycol, and shellac glaze.