Dofetilide Prescribing Information
To minimize the risk of induced arrhythmia, patients initiated or re-initiated on dofetilide should be placed for a minimum of 3 days in a facility that can provide calculations of creatinine clearance, continuous electrocardiographic monitoring, and cardiac resuscitation. For detailed instructions regarding dose selection, see
- Therapy with dofetilide must be initiated (and, if necessary, re-initiated) in a setting that provides continuous electrocardiographic (ECG) monitoring and in the presence of personnel trained in the management of serious ventricular arrhythmias. Patients should continue to be monitored in this way for a minimum of three days. Additionally, patients should not be discharged within 12 hours of electrical or pharmacological conversion to normal sinus rhythm.The dose of dofetilide must be individualized according to calculated creatinine clearance and QTc. (QT interval should be used if the heart rate is <60 beats per minute. There are no data on use of dofetilide when the heart rate is <50 beats per minute.)The usual recommended dose of dofetilide is 500 mcg BID, as modified by the dosing algorithm described below. For consideration of a lower dose, seeSpecial Considerationsbelow.
- Serum potassium should be maintained within the normal range before dofetilide treatment is initiated and should be maintained within the normal range while the patient remains on dofetilide therapy. (SeeWARNINGS, Hypokalemia and Potassium-Depleting Diuretics). In clinical trials, potassium levels were generally maintained above 3.6 to 4 mEq/L.
- Patients with atrial fibrillation should be anticoagulated according to usual medical practice prior to electrical or pharmacological cardioversion. Anticoagulant therapy may be continued after cardioversion according to usual medical practice for the treatment of people with AF. Hypokalemia should be corrected before initiation of dofetilide therapy (seeWARNINGS, Ventricular Arrhythmia).
- Patients to be discharged on dofetilide therapy from an inpatient setting as described above must have an adequate supply of dofetilide, at the patient’s individualized dose, to allow uninterrupted dosing until the patient can fill a dofetilide prescription.
creatinine clearance (female) =
When serum creatinine is given in µmol/L, divide the value by 88.4 (1 mg/dL = 88.4 µmol/L).
| Calculated Creatinine Clearance | Dofetilide Dose |
| >60 mL/min | 500 mcg twice daily |
| 40 to 60 mL/min | 250 mcg twice daily |
| 20 to <40 mL/min | 125 mcg twice daily |
| <20 mL/min | Dofetilide is contraindicated in these patients |
If the Starting Dose Based on Creatinine Clearance is: | Then the Adjusted Dose (for QTc or QT Prolongation) is: |
| 500 mcg twice daily | 250 mcg twice daily |
| 250 mcg twice daily | 125 mcg twice daily |
| 125 mcg twice daily | 125 mcg once a day |
NOTE: If at any time after the second dose of dofetilide is given the QTc or QT is greater than 500 msec (550 msec in patients with ventricular conduction abnormalities), dofetilide should be discontinued.
The steps described above are summarized in the following diagram:
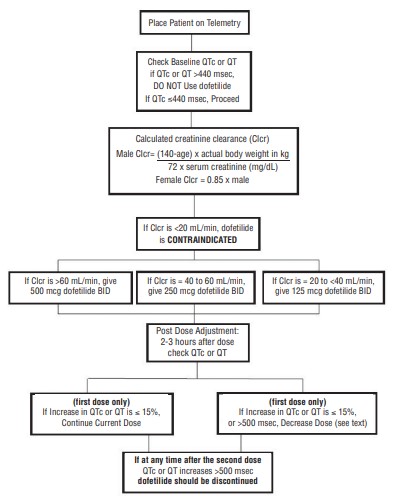
Renal function and QTc or QT (if heart rate is less than 60 beats per minute) should be re-evaluated every three months or as medically warranted. If QTc or QT exceeds 500 milliseconds (550 msec in patients with ventricular conduction abnormalities), dofetilide therapy should be discontinued and patients should be carefully monitored until QTc or QT returns to baseline levels. If renal function deteriorates, adjust dose as described in Initiation of Dofetilide Therapy, Step 3.
Consideration of a Dose Lower than that Determined by the Algorithm: The dosing algorithm shown above should be used to determine the individualized dose of dofetilide. In clinical trials (see
The maximum recommended dose in patients with a calculated creatinine clearance greater than 60 mL/min is 500 mcg BID; doses greater than 500 mcg BID have been associated with an increased incidence of Torsade de Pointes.
A patient who misses a dose should NOT double the next dose. The next dose should be taken at the usual time.
Cardioversion: If patients do not convert to normal sinus rhythm within 24 hours of initiation of dofetilide therapy, electrical conversion should be considered. Patients continuing on dofetilide after successful electrical cardioversion should continue to be monitored by electrocardiography for 12 hours post cardioversion, or a minimum of 3 days after initiation of dofetilide therapy, whichever is greater.
Before initiating dofetilide therapy, previous antiarrhythmic therapy should be withdrawn under careful monitoring for a minimum of three (3) plasma half-lives. Because of the unpredictable pharmacokinetics of amiodarone, dofetilide should not be initiated following amiodarone therapy until amiodarone plasma levels are below 0.3 mcg/mL or until amiodarone has been withdrawn for at least three months.
If dofetilide needs to be discontinued to allow dosing of other potentially interacting drug(s), a washout period of at least two days should be followed before starting the other drug(s).

- Therapy with dofetilide must be initiated (and, if necessary, re-initiated) in a setting that provides continuous electrocardiographic (ECG) monitoring and in the presence of personnel trained in the management of serious ventricular arrhythmias. Patients should continue to be monitored in this way for a minimum of three days. Additionally, patients should not be discharged within 12 hours of electrical or pharmacological conversion to normal sinus rhythm.The dose of dofetilide must be individualized according to calculated creatinine clearance and QTc. (QT interval should be used if the heart rate is <60 beats per minute. There are no data on use of dofetilide when the heart rate is <50 beats per minute.)The usual recommended dose of dofetilide is 500 mcg BID, as modified by the dosing algorithm described below. For consideration of a lower dose, seeSpecial Considerationsbelow.
- Serum potassium should be maintained within the normal range before dofetilide treatment is initiated and should be maintained within the normal range while the patient remains on dofetilide therapy. (See ). In clinical trials, potassium levels were generally maintained above 3.6 to 4 mEq/L.Drug-Drug Interactions(seeCONTRAINDICATIONS)
Because there is a linear relationship between dofetilide plasma concentration and QTc, concomitant drugs that interfere with the metabolism or renal elimination of dofetilide may increase the risk of arrhythmia (Torsade de Pointes). Dofetilide is metabolized to a small degree by the CYP3A4 isoenzyme of the cytochrome P450 system and an inhibitor of this system could increase systemic dofetilide exposure. More important, dofetilide is eliminated by cationic renal secretion, and three inhibitors of this process have been shown to increase systemic dofetilide exposure. The magnitude of the effect on renal elimination by cimetidine, trimethoprim, and ketoconazole (all contraindicated concomitant uses with dofetilide) suggests that all renal cation transport inhibitors should be contraindicated.
- Patients with atrial fibrillation should be anticoagulated according to usual medical practice prior to electrical or pharmacological cardioversion. Anticoagulant therapy may be continued after cardioversion according to usual medical practice for the treatment of people with AF. Hypokalemia should be corrected before initiation of dofetilide therapy (see ).Drug-Drug Interactions(seeCONTRAINDICATIONS)
Because there is a linear relationship between dofetilide plasma concentration and QTc, concomitant drugs that interfere with the metabolism or renal elimination of dofetilide may increase the risk of arrhythmia (Torsade de Pointes). Dofetilide is metabolized to a small degree by the CYP3A4 isoenzyme of the cytochrome P450 system and an inhibitor of this system could increase systemic dofetilide exposure. More important, dofetilide is eliminated by cationic renal secretion, and three inhibitors of this process have been shown to increase systemic dofetilide exposure. The magnitude of the effect on renal elimination by cimetidine, trimethoprim, and ketoconazole (all contraindicated concomitant uses with dofetilide) suggests that all renal cation transport inhibitors should be contraindicated.
- Patients to be discharged on dofetilide therapy from an inpatient setting as described above must have an adequate supply of dofetilide, at the patient’s individualized dose, to allow uninterrupted dosing until the patient can fill a dofetilide prescription.
Dofetilide is contraindicated in patients with congenital or acquired long QT syndromes. Dofetilide should not be used in patients with a baseline QT interval or QTc >440 msec (500 msec in patients with ventricular conduction abnormalities). Dofetilide is also contraindicated in patients with severe renal impairment (calculated creatinine clearance <20 mL/min).
The concomitant use of verapamil or the cation transport system inhibitors cimetidine, trimethoprim (alone or in combination with sulfamethoxazole), or ketoconazole with dofetilide is contraindicated (see
The risk of dofetilide induced ventricular arrhythmia was assessed in three ways in clinical studies: 1) by description of the QT interval and its relation to the dose and plasma concentration of dofetilide; 2) by observing the frequency of TdP in dofetilide-treated patients according to dose; 3) by observing the overall mortality rate in patients with atrial fibrillation and in patients with structural heart disease.
Relation of QT Interval to Dose
The QT interval increases linearly with increasing DOFETILIDE dose (see Figures 1 and 2 in
Frequency of Torsade de Pointes
In the supraventricular arrhythmia population (patients with AF and other supraventricular arrhythmias), the overall incidence of Torsade de Pointes was 0.8%. The frequency of TdP by dose is shown in Table 4. There were no cases of TdP on placebo.
| Dofetilide Dose | |||||
|---|---|---|---|---|---|
| <250 mcg BID | 250 mcg BID | >250 to 500 mcg BID | >500 mcg BID | All Doses | |
| Number of Patients | 217 | 388 | 703 | 38 | 1346 |
| Torsade de Pointes | 0 | 1 (0.3%) | 6 (0.9%) | 4 (10.5%) | 11 (0.8%) |
As shown in Table 5, the rate of TdP was reduced when patients were dosed according to their renal function (see
| Total | Before | After | |
|---|---|---|---|
| Population: | n/N % | n/N % | n/N % |
| Supraventricular Arrhythmias | 11/1346 (0.8%) | 6/193 (3.1%) | 5/1153 (0.4%) |
| DIAMOND CHF | 25/762 (3.3%) | 7/148 (4.7%) | 18/614 (2.9%) |
| DIAMOND MI | 7/749 (0.9%) | 3/101 (3.0%) | 4/648 (0.6%) |
| DIAMOND AF | 4/249 (1.6%) | 0/43 (0%) | 4/206 (1.9%) |
The majority of the episodes of TdP occurred within the first three days of dofetilide therapy (10/11 events in the studies of patients with supraventricular arrhythmias; 19/25 and 4/7 events in DIAMOND CHF and DIAMOND MI, respectively; 2/4 events in the DIAMOND AF subpopulation).
Mortality
In a pooled survival analysis of patients in the supraventricular arrhythmia population (low prevalence of structural heart disease), deaths occurred in 0.9% (12/1346) of patients receiving dofetilide and 0.4% (3/677) in the placebo group. Adjusted for duration of therapy, primary diagnosis, age, gender, and prevalence of structural heart disease, the point estimate of the hazard ratio for the pooled studies (dofetilide/placebo) was 1.1 (95% CI: 0.3, 4.3). The DIAMOND CHF and MI trials examined mortality in patients with structural heart disease (ejection fraction 35%). In these large, double-blind studies, deaths occurred in 36% (541/1511) of dofetilide patients and 37% (560/1517) of placebo patients. In an analysis of 506 DIAMOND patients with atrial fibrillation/flutter at baseline, one year mortality on dofetilide was 31% vs. 32% on placebo (see
Because of the small number of events, an excess mortality due to dofetilide cannot be ruled out with confidence in the pooled survival analysis of placebo-controlled trials in patients with supraventricular arrhythmias. However, it is reassuring that in two large placebo-controlled mortality studies in patients with significant heart disease (DIAMOND CHF/MI), there were no more deaths in dofetilide-treated patients than in patients given placebo (see
Because there is a linear relationship between dofetilide plasma concentration and QTc, concomitant drugs that interfere with the metabolism or renal elimination of dofetilide may increase the risk of arrhythmia (Torsade de Pointes). Dofetilide is metabolized to a small degree by the CYP3A4 isoenzyme of the cytochrome P450 system and an inhibitor of this system could increase systemic dofetilide exposure. More important, dofetilide is eliminated by cationic renal secretion, and three inhibitors of this process have been shown to increase systemic dofetilide exposure. The magnitude of the effect on renal elimination by cimetidine, trimethoprim, and ketoconazole (all contraindicated concomitant uses with dofetilide) suggests that all renal cation transport inhibitors should be contraindicated.
Hypokalemia or hypomagnesemia may occur with administration of potassium-depleting diuretics, increasing the potential for Torsade de Pointes. Potassium levels should be within the normal range prior to administration of dofetilide and maintained in the normal range during administration of dofetilide (see
The use of dofetilide in conjunction with other drugs that prolong the QT interval has not been studied and is not recommended. Such drugs include phenothiazines, cisapride, bepridil, tricyclic antidepressants, certain oral macrolides, and certain fluoroquinolones. Class I or Class III antiarrhythmic agents should be withheld for at least three half-lives prior to dosing with dofetilide. In clinical trials, dofetilide was administered to patients previously treated with oral amiodarone only if serum amiodarone levels were below 0.3 mg/L or amiodarone had been withdrawn for at least three months.
The overall systemic clearance of dofetilide is decreased and plasma concentration increased with decreasing creatinine clearance. The dose of dofetilide must be adjusted based on creatinine clearance (see
After adjustment for creatinine clearance, no additional dose adjustment is required for patients with mild or moderate hepatic impairment. Patients with severe hepatic impairment have not been studied. Dofetilide should be used with particular caution in these patients.
Animal and human studies have not shown any adverse effects of dofetilide on conduction velocity. No effect on AV nodal conduction following dofetilide treatment was noted in normal volunteers and in patients with 1st degree heart block. Patients with sick sinus syndrome or with 2nd or 3rd degree heart block were not included in the Phase 3 clinical trials unless a functioning pacemaker was present. Dofetilide has been used safely in conjunction with pacemakers (53 patients in DIAMOND studies, 136 in trials in patients with ventricular and supraventricular arrhythmias).
The concomitant use of hydrochlorothiazide (alone or in combinations such as with triamterene) with dofetilide is contraindicated (see
The overall systemic clearance of dofetilide is decreased and plasma concentration increased with decreasing creatinine clearance. The dose of dofetilide must be adjusted based on creatinine clearance (see
After adjustment for creatinine clearance, no additional dose adjustment is required for patients with mild or moderate hepatic impairment. Patients with severe hepatic impairment have not been studied. Dofetilide should be used with particular caution in these patients.
Animal and human studies have not shown any adverse effects of dofetilide on conduction velocity. No effect on AV nodal conduction following dofetilide treatment was noted in normal volunteers and in patients with 1st degree heart block. Patients with sick sinus syndrome or with 2nd or 3rd degree heart block were not included in the Phase 3 clinical trials unless a functioning pacemaker was present. Dofetilide has been used safely in conjunction with pacemakers (53 patients in DIAMOND studies, 136 in trials in patients with ventricular and supraventricular arrhythmias).
Dofetilide is also contraindicated in patients with a known hypersensitivity to the drug.
The dofetilide clinical program involved approximately 8,600 patients in 130 clinical studies of normal volunteers and patients with supraventricular and ventricular arrhythmias. Dofetilide was administered to 5,194 patients, including two large, placebo-controlled mortality trials (DIAMOND CHF and DIAMOND MI) in which 1,511 patients received dofetilide for up to three years.
In the following section, adverse reaction data for cardiac arrhythmias and non-cardiac adverse reactions are presented separately for patients included in the supraventricular arrhythmia development program and for patients included in the DIAMOND CHF and MI mortality trials (see
The two DIAMOND studies were 3-year trials comparing the effects of dofetilide and placebo on mortality and morbidity in patients with impaired left ventricular function (ejection fraction
In both DIAMOND studies, patients were randomized to 500 mcg BID of dofetilide, but this was reduced to 250 mcg BID if calculated creatinine clearance was 40 to 60 mL/min, if patients had AF, or if QT interval prolongation (>550 msec or >20% increase from baseline) occurred after dosing. Dose reductions for reduced calculated creatinine clearance occurred in 47% and 45% of DIAMOND CHF and MI patients, respectively. Dose reductions for increased QT interval or QTc occurred in 5% and 7% of DIAMOND CHF and MI patients, respectively. Increased QT interval or QTc (>550 msec or >20% increase from baseline) resulted in discontinuation of 1.8% of patients in DIAMOND CHF and 2.5% of patients in DIAMOND MI.
The probability of survival at one year was 79% (95% CI: 76% to 82%) in the dofetilide group and 77% (95% CI: 74% to 80%) in the placebo group. Cardiac and arrhythmic mortality showed a similar result. Torsade de Pointes occurred in 7/749 patients (0.9%) receiving dofetilide. Of these, 4 cases occurred within the first 3 days of dosing and 3 cases occurred between Day 4 and the conclusion of the study. In all, 371/749 (50%) of patients on dofetilide and 419/761 (55%) on placebo required hospitalization. Of these, 200/749 (27%) of patients on dofetilide and 205/761 (27%) on placebo required hospitalization because of worsening heart failure.
Of the 506 patients in the DIAMOND studies who had atrial fibrillation or flutter at baseline, 12% of patients in the dofetilide group and 2% of patients in the placebo group had converted to normal sinus rhythm after one month. In those patients converted to normal sinus rhythm, 79% of the dofetilide group and 42% of the placebo group remained in normal sinus rhythm for one year.
In the DIAMOND studies, although Torsade de Pointes occurred more frequently in the dofetilide-treated patients (see
In studies of patients with supraventricular arrhythmias, a total of 1,346 and 677 patients were exposed to dofetilide and placebo for 551 and 207 patient years, respectively. A total of 8.7% of patients in the dofetilide groups were discontinued from clinical trials due to adverse events compared to 8% in the placebo groups. The most frequent reason for discontinuation (>1%) was ventricular tachycardia (2% on dofetilide vs. 1.3% on placebo). The most frequent adverse events were headache, chest pain, and dizziness.
Because there is a linear relationship between dofetilide plasma concentration and QTc, concomitant drugs that interfere with the metabolism or renal elimination of dofetilide may increase the risk of arrhythmia (Torsade de Pointes). Dofetilide is metabolized to a small degree by the CYP3A4 isoenzyme of the cytochrome P450 system and an inhibitor of this system could increase systemic dofetilide exposure. More important, dofetilide is eliminated by cationic renal secretion, and three inhibitors of this process have been shown to increase systemic dofetilide exposure. The magnitude of the effect on renal elimination by cimetidine, trimethoprim, and ketoconazole (all contraindicated concomitant uses with dofetilide) suggests that all renal cation transport inhibitors should be contraindicated.
- Therapy with dofetilide must be initiated (and, if necessary, re-initiated) in a setting that provides continuous electrocardiographic (ECG) monitoring and in the presence of personnel trained in the management of serious ventricular arrhythmias. Patients should continue to be monitored in this way for a minimum of three days. Additionally, patients should not be discharged within 12 hours of electrical or pharmacological conversion to normal sinus rhythm.The dose of dofetilide must be individualized according to calculated creatinine clearance and QTc. (QT interval should be used if the heart rate is <60 beats per minute. There are no data on use of dofetilide when the heart rate is <50 beats per minute.)The usual recommended dose of dofetilide is 500 mcg BID, as modified by the dosing algorithm described below. For consideration of a lower dose, seeSpecial Considerationsbelow.
- Serum potassium should be maintained within the normal range before dofetilide treatment is initiated and should be maintained within the normal range while the patient remains on dofetilide therapy. (SeeWARNINGS, Hypokalemia and Potassium-Depleting Diuretics). In clinical trials, potassium levels were generally maintained above 3.6 to 4 mEq/L.
- Patients with atrial fibrillation should be anticoagulated according to usual medical practice prior to electrical or pharmacological cardioversion. Anticoagulant therapy may be continued after cardioversion according to usual medical practice for the treatment of people with AF. Hypokalemia should be corrected before initiation of dofetilide therapy (seeWARNINGS, Ventricular Arrhythmia).
- Patients to be discharged on dofetilide therapy from an inpatient setting as described above must have an adequate supply of dofetilide, at the patient’s individualized dose, to allow uninterrupted dosing until the patient can fill a dofetilide prescription.
creatinine clearance (female) =
When serum creatinine is given in µmol/L, divide the value by 88.4 (1 mg/dL = 88.4 µmol/L).
| Calculated Creatinine Clearance | Dofetilide Dose |
| >60 mL/min | 500 mcg twice daily |
| 40 to 60 mL/min | 250 mcg twice daily |
| 20 to <40 mL/min | 125 mcg twice daily |
| <20 mL/min | Dofetilide is contraindicated in these patients |
If the Starting Dose Based on Creatinine Clearance is: | Then the Adjusted Dose (for QTc or QT Prolongation) is: |
| 500 mcg twice daily | 250 mcg twice daily |
| 250 mcg twice daily | 125 mcg twice daily |
| 125 mcg twice daily | 125 mcg once a day |
NOTE: If at any time after the second dose of dofetilide is given the QTc or QT is greater than 500 msec (550 msec in patients with ventricular conduction abnormalities), dofetilide should be discontinued.
The steps described above are summarized in the following diagram:

Renal function and QTc or QT (if heart rate is less than 60 beats per minute) should be re-evaluated every three months or as medically warranted. If QTc or QT exceeds 500 milliseconds (550 msec in patients with ventricular conduction abnormalities), dofetilide therapy should be discontinued and patients should be carefully monitored until QTc or QT returns to baseline levels. If renal function deteriorates, adjust dose as described in Initiation of Dofetilide Therapy, Step 3.
Consideration of a Dose Lower than that Determined by the Algorithm: The dosing algorithm shown above should be used to determine the individualized dose of dofetilide. In clinical trials (see
The maximum recommended dose in patients with a calculated creatinine clearance greater than 60 mL/min is 500 mcg BID; doses greater than 500 mcg BID have been associated with an increased incidence of Torsade de Pointes.
A patient who misses a dose should NOT double the next dose. The next dose should be taken at the usual time.
Cardioversion: If patients do not convert to normal sinus rhythm within 24 hours of initiation of dofetilide therapy, electrical conversion should be considered. Patients continuing on dofetilide after successful electrical cardioversion should continue to be monitored by electrocardiography for 12 hours post cardioversion, or a minimum of 3 days after initiation of dofetilide therapy, whichever is greater.
Before initiating dofetilide therapy, previous antiarrhythmic therapy should be withdrawn under careful monitoring for a minimum of three (3) plasma half-lives. Because of the unpredictable pharmacokinetics of amiodarone, dofetilide should not be initiated following amiodarone therapy until amiodarone plasma levels are below 0.3 mcg/mL or until amiodarone has been withdrawn for at least three months.
If dofetilide needs to be discontinued to allow dosing of other potentially interacting drug(s), a washout period of at least two days should be followed before starting the other drug(s).

| Dofetilide Dose | Placebo | ||||
|---|---|---|---|---|---|
| Arrhythmia event: | <250 mcg BID N=217 | 250 mcg BID N=388 | >250 to 500 mcg BID N=703 | >500 mcg BID N=38 | N=677 |
| Ventricular arrhythmias *† | 3.7% | 2.6% | 3.4% | 15.8% | 2.7% |
| Ventricular fibrillation | 0 | 0.3% | 0.4% | 2.6% | 0.1% |
| Ventricular tachycardia † | 3.7% | 2.6% | 3.3% | 13.2% | 2.5% |
| Torsade de Pointes | 0 | 0.3% | 0.9% | 10.5% | 0 |
| Various forms of block | |||||
| AV block | 0.9% | 1.5% | 0.4% | 0 | 0.3% |
| Bundle branch block | 0 | 0.5% | 0.1% | 0 | 0.1% |
| Heart block | 0 | 0.5% | 0.1% | 0 | 0.1% |
* Patients with more than one arrhythmia are counted only once in this category.
^ Ventricular arrhythmias and ventricular tachycardia include all cases of Torsade de Pointes
In the DIAMOND trials, a total of 1,511 patients were exposed to dofetilide for 1757 patient years. The incidence of Torsade de Pointes was 3.3% in CHF patients and 0.9% in patients with a recent MI.
Table 7 shows the incidence of serious arrhythmias and conduction disturbances reported as adverse events in the DIAMOND subpopulation that had AF at entry to these trials.
| Dofetilide | Placebo | |
|---|---|---|
| N=249 | N=257 | |
| Ventricular arrhythmias *† | 14.5% | 13.6% |
| Ventricular fibrillation | 4.8% | 3.1% |
| Ventricular tachycardia † | 12.4% | 11.3% |
| Torsade de Pointes | 1.6% | 0 |
| Various forms of block | ||
| AV block | 0.8% | 2.7% |
| (Left) bundle branch block | 0 | 0.4% |
| Heart block | 1.2% | 0.8% |
* Patients with more than one arrhythmia are counted only once in this category.
^ Ventricular arrhythmias and ventricular tachycardia include all cases of Torsade de Pointes.
| Dofetilide | Placebo | |
|---|---|---|
| Adverse Event | % | % |
| headache | 11 | 9 |
| chest pain | 10 | 7 |
| dizziness | 8 | 6 |
| respiratory tract infection | 7 | 5 |
| dyspnea | 6 | 5 |
| nausea | 5 | 4 |
| flu syndrome | 4 | 2 |
| insomnia | 4 | 3 |
| accidental injury | 3 | 1 |
| back pain | 3 | 2 |
| procedure (medical/surgical/health service) | 3 | 2 |
| diarrhea | 3 | 2 |
| rash | 3 | 2 |
| abdominal pain | 3 | 2 |
Adverse events reported at a rate >2% but no more frequently on dofetilide than on placebo were: angina pectoris, anxiety, arthralgia, asthenia, atrial fibrillation, complications (application, injection, incision, insertion, or device), hypertension, pain, palpitation, peripheral edema, supraventricular tachycardia, sweating, urinary tract infection, ventricular tachycardia.
The following adverse events have been reported with a frequency of 2% and numerically more frequently with dofetilide than placebo in patients with supraventricular arrhythmias: angioedema, bradycardia, cerebral ischemia, cerebrovascular accident, edema, facial paralysis, flaccid paralysis, heart arrest, increased cough, liver damage, migraine, myocardial infarct, paralysis, paresthesia, sudden death, and syncope.
The incidences of clinically significant laboratory test abnormalities in patients with supraventricular arrhythmias were similar for patients on dofetilide and those on placebo. No clinically relevant effects were noted in serum alkaline phosphatase, serum GGT, LDH, AST, ALT, total bilirubin, total protein, blood urea nitrogen, creatinine, serum electrolytes (calcium, chloride, glucose, magnesium, potassium, sodium), or creatine kinase. Similarly, no clinically relevant effects were observed in hematologic parameters.
In the DIAMOND population, adverse events other than those related to the post-infarction and heart failure patient population were generally similar to those seen in the supraventricular arrhythmia groups.
Dofetilide is an antiarrhythmic drug with Class III (cardiac action potential duration prolonging) properties. Its empirical formula is C19H27N3O5S2 and it has a molecular weight of 441.6. The structural formula is
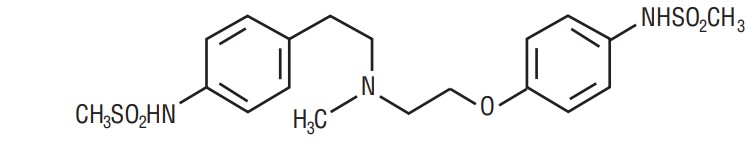
The chemical name for dofetilide is:
N-[4-[2-[methyl[2-[4-[(methylsulfonyl)amino]phenoxy]ethyl]amino]ethyl]phenyl]-methanesulfonamide.
Dofetilide is a white to off-white powder. It is very slightly soluble in water and propan-2-ol and is soluble in 0.1M aqueous sodium hydroxide, acetone, and aqueous 0.1M hydrochloric acid.
Dofetilide capsules contain the following inactive ingredients: colloidal silicon dioxide, corn starch, gelatin, magnesium stearate, microcrystalline cellulose and titanium dioxide. The 125 mcg capsules contain FD & C Yellow # 6 and D & C yellow # 10. The 250 mcg and 500 mcg capsules contain FD & C Yellow # 6 and FD& C Red # 40. Dofetilide is supplied for oral administration in three dosage strengths: 125 mcg (0.125 mg) orange and white capsules, 250 mcg (0.25 mg) peach capsules, and 500 mcg (0.5 mg) peach and white capsules. In addition, capsule printing ink contains ammonium hydroxide, black iron oxide, propylene glycol, and shellac glaze.
Dofetilide capsules are supplied as:
Hard gelatin capsule, orange opaque cap printed with “G125” and white opaque body printed with “024” contains white to off-white powder.
Bottles of 60 Capsules NDC 76282-755-60
Hard gelatin capsule, peach opaque cap printed with “G250” and peach opaque body printed with “025” contains white to off-white powder.
Bottles of 60 Capsules NDC 76282-756-60
Hard gelatin capsule, peach opaque cap printed with “G500” and white opaque body printed with “026” contains white to off-white powder.
Bottles of 60 Capsules NDC 76282-757-60