Dofetilide Prescribing Information
To minimize the risk of induced arrhythmia, patients initiated or re-initiated on dofetilide capsules should be placed for a minimum of 3 days in a facility that can provide calculations of creatinine clearance, continuous electrocardiographic monitoring, and cardiac resuscitation. For detailed instructions regarding dose selection, see
Dofetilide capsules are indicated for the maintenance of normal sinus rhythm (delay in time to recurrence of atrial fibrillation/atrial flutter [AF/AFl]) in patients with atrial fibrillation/atrial flutter of greater than one week duration who have been converted to normal sinus rhythm. Because dofetilide can cause life threatening ventricular arrhythmias, it should be reserved for patients in whom atrial fibrillation/atrial flutter is highly symptomatic.
In general, antiarrhythmic therapy for atrial fibrillation/atrial flutter aims to prolong the time in normal sinus rhythm. Recurrence is expected in some patients (see
Dofetilide capsules are indicated for the conversion of atrial fibrillation and atrial flutter to normal sinus rhythm.
Dofetilide capsules have not been shown to be effective in patients with paroxysmal atrial fibrillation.
- Therapy with dofetilide must be initiated (and, if necessary, re-initiated) in a setting that provides continuous electrocardiographic (ECG) monitoring and in the presence of personnel trained in the management of serious ventricular arrhythmias. Patients should continue to be monitored in this way for a minimum of three days. Additionally, patients should not be discharged within 12 hours of electrical or pharmacological conversion to normal sinus rhythm.
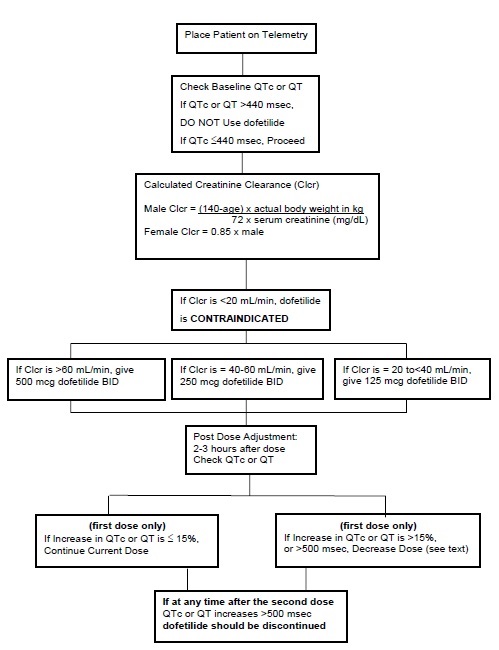
- The dose of dofetilide must be individualized according to calculated creatinine clearance and QTc. (QT interval should be used if the heart rate is <60 beats per minute. There are no data on use of dofetilide when the heart rate is <50 beats per minute.) The usual recommended dose of dofetilide is 500 mcg BID, as modified by the dosing algorithm described below. For consideration of a lower dose, see Special Considerations below.
- Serum potassium should be maintained within the normal range before dofetilide treatment is initiated and should be maintained within the normal range while the patient remains on dofetilide therapy. (See WARNINGS, Hypokalemia and Potassium-Depleting Diuretics). In clinical trials, potassium levels were generally maintained above 3.6 to 4.0 mEq/L.
- Patients with atrial fibrillation should be anticoagulated according to usual medical practice prior to electrical or pharmacological cardioversion. Anticoagulant therapy may be continued after cardioversion according to usual medical practice for the treatment of people with AF. Hypokalemia should be corrected before initiation of dofetilide therapy (see WARNINGS, Ventricular Arrhythmia).
- Patients to be discharged on dofetilide therapy from an inpatient setting as described above must have an adequate supply of dofetilide capsules, at the patient’s individualized dose, to allow uninterrupted dosing until the patient can fill a dofetilide prescription.
Initiation of Dofetilide Therapy
creatinine clearance (male) =
72 x serum creatinine (mg/dL)
creatinine clearance (female) =
72 x serum creatinine (mg/dL)
When serum creatinine is given in μmol/L, divide the value by 88.4 (1 mg/dL = 88.4 μmol/L).
Calculated Creatinine Clearance | Dofetilide Dose |
| >60 mL/min | 500 mcg twice daily |
| 40 to 60 mL/min | 250 mcg twice daily |
| 20 to <40 mL/min | 125 mcg twice daily |
| <20 mL/min | Dofetilide is contraindicated in these patients |
If the Starting Dose Based on Creatinine Clearance is: | Then the Adjusted Dose (for QTc or QT Prolongation) is: |
| 500 mcg twice daily | 250 mcg twice daily |
| 250 mcg twice daily | 125 mcg twice daily |
| 125 mcg twice daily | 125 mcg once a day |
NOTE: If at any time after the second dose of dofetilide is given the QTc or QT is greater than 500 msec (550 msec in patients with ventricular conduction abnormalities), dofetilide should be discontinued.
The steps described above are summarized in the following diagram:
Renal function and QTc or QT (if heart rate is less than 60 beats per minute) should be re-evaluated every three months or as medically warranted. If QTc or QT exceeds 500 milliseconds (550 msec in patients with ventricular conduction abnormalities), dofetilide therapy should be discontinued and patients should be carefully monitored until QTc or QT returns to baseline levels. If renal function deteriorates, adjust dose as described in Initiation of Dofetilide Therapy, Step 3.
Consideration of a Dose Lower than that Determined by the Algorithm:
The dosing algorithm shown above should be used to determine the individualized dose of dofetilide. In clinical trials (seeThe maximum recommended dose in patients with a calculated creatinine clearance greater than 60 mL/min is 500 mcg BID; doses greater than 500 mcg BID have been associated with an increased incidence of Torsade de Pointes.
A patient who misses a dose should NOT double the next dose. The next dose should be taken at the usual time.
Cardioversion: If patients do not convert to normal sinus rhythm within 24 hours of initiation of dofetilide therapy, electrical conversion should be considered. Patients continuing on dofetilide after successful electrical cardioversion should continue to be monitored by electrocardiography for 12 hours post cardioversion, or a minimum of 3 days after initiation of dofetilide therapy, whichever is greater.
Before initiating dofetilide therapy, previous antiarrhythmic therapy should be withdrawn under careful monitoring for a minimum of three (3) plasma half-lives. Because of the unpredictable pharmacokinetics of amiodarone, dofetilide should not be initiated following amiodarone therapy until amiodarone plasma levels are below 0.3 mcg/mL or until amiodarone has been withdrawn for at least three months.
If dofetilide needs to be discontinued to allow dosing of other potentially interacting drug(s), a washout period of at least two days should be followed before starting the other drug(s).
Dofetilide capsules are contraindicated in patients with congenital or acquired long QT syndromes. Dofetilide capsules should not be used in patients with a baseline QT interval or QTc >440 msec (500 msec in patients with ventricular conduction abnormalities). Dofetilide capsules are also contraindicated in patients with severe renal impairment (calculated creatinine clearance <20 mL/min).
The concomitant use of verapamil or the cation transport system inhibitors cimetidine, trimethoprim (alone or in combination with sulfamethoxazole), or ketoconazole with dofetilide capsules is contraindicated (see
The concomitant use of hydrochlorothiazide (alone or in combinations such as with triamterene) with dofetilide capsules is contraindicated (see
Dofetilide capsules are also contraindicated in patients with a known hypersensitivity to the drug.
The dofetilide clinical program involved approximately 8,600 patients in 130 clinical studies of normal volunteers and patients with supraventricular and ventricular arrhythmias. Dofetilide was administered to 5,194 patients, including two large, placebo-controlled mortality trials (DIAMOND CHF and DIAMOND MI) in which 1,511 patients received dofetilide for up to three years.
In the following section, adverse reaction data for cardiac arrhythmias and non-cardiac adverse reactions are presented separately for patients included in the supraventricular arrhythmia development program and for patients included in the DIAMOND CHF and MI mortality trials (see
In studies of patients with supraventricular arrhythmias, a total of 1,346 and 677 patients were exposed to dofetilide and placebo for 551 and 207 patient years, respectively. A total of 8.7% of patients in the dofetilide groups were discontinued from clinical trials due to adverse events compared to 8.0% in the placebo groups. The most frequent reason for discontinuation (>1%) was ventricular tachycardia (2.0% on dofetilide vs. 1.3% on placebo). The most frequent adverse events were headache, chest pain, and dizziness.
| * Patients with more than one arrhythmia are counted only once in this category. ^ Ventricular arrhythmias and ventricular tachycardia include all cases of Torsade de Pointes. | |||||
| | Dofetilide Dose | Placebo | |||
| Arrhythmia event: | <250 mcg BID N=217 | 250 mcg BID N=388 | >250 to 500 mcg BID N=703 | >500 mcg BID N=38 | N=677 |
| Ventricular arrhythmias *^ | 3.7% | 2.6% | 3.4% | 15.8% | 2.7% |
| Ventricular fibrillation | 0 | 0.3% | 0.4% | 2.6% | 0.1% |
| Ventricular tachycardia^ | 3.7% | 2.6% | 3.3% | 13.2% | 2.5% |
| Torsade de Pointes | 0 | 0.3% | 0.9% | 10.5% | 0 |
| Various forms of block | | | | | |
| AV block | 0.9% | 1.5% | 0.4% | 0 | 0.3% |
| Bundle branch block | 0 | 0.5% | 0.1% | 0 | 0.1% |
| Heart block | 0 | 0.5% | 0.1% | 0 | 0.1% |
In the DIAMOND trials, a total of 1,511 patients were exposed to dofetilide for 1757 patient years. The incidence of Torsade de Pointes was 3.3% in CHF patients and 0.9% in patients with a recent MI.
Table 7 shows the incidence of serious arrhythmias and conduction disturbances reported as adverse events in the DIAMOND subpopulation that had AF at entry to these trials.
| | Dofetilide | Placebo |
| | N=249 | N=257 |
| Ventricular arrhythmias*^ | 14.5% | 13.6% |
| Ventricular fibrillation | 4.8% | 3.1% |
| Ventricular tachycardia^ | 12.4% | 11.3% |
| Torsade de Pointes | 1.6% | 0 |
| Various forms of block | | |
| AV block | 0.8% | 2.7% |
| (Left) bundle branch block | 0 | 0.4% |
| Heart block | 1.2% | 0.8% |
* Patients with more than one arrhythmia are counted only once in this category.
^ Ventricular arrhythmias and ventricular tachycardia include all cases of Torsade de Pointes.
| | Dofetilide | Placebo |
| Adverse Event | % | % |
| headache | 11 | 9 |
| chest pain | 10 | 7 |
| dizziness | 8 | 6 |
| respiratory tract infection | 7 | 5 |
| dyspnea | 6 | 5 |
| nausea | 5 | 4 |
| flu syndrome | 4 | 2 |
| insomnia | 4 | 3 |
| accidental injury | 3 | 1 |
| back pain | 3 | 2 |
| procedure (medical/surgical/health service) | 3 | 2 |
| diarrhea | 3 | 2 |
| rash | 3 | 2 |
| abdominal pain | 3 | 2 |
Adverse events reported at a rate >2% but no more frequently on dofetilide than on placebo were: angina pectoris, anxiety, arthralgia, asthenia, atrial fibrillation, complications (application, injection, incision, insertion, or device), hypertension, pain, palpitation, peripheral edema, supraventricular tachycardia, sweating, urinary tract infection, ventricular tachycardia.
The following adverse events have been reported with a frequency of
The incidences of clinically significant laboratory test abnormalities in patients with supraventricular arrhythmias were similar for patients on dofetilide and those on placebo. No clinically relevant effects were noted in serum alkaline phosphatase, serum GGT, LDH, AST, ALT, total bilirubin, total protein, blood urea nitrogen, creatinine, serum electrolytes (calcium, chloride, glucose, magnesium, potassium, sodium), or creatine kinase. Similarly, no clinically relevant effects were observed in hematologic parameters.
In the DIAMOND population, adverse events other than those related to the post-infarction and heart failure patient population were generally similar to those seen in the supraventricular arrhythmia groups.
Cimetidine: (see
Verapamil: (see
Ketoconazole: (see
Trimethoprim Alone or in Combination with Sulfamethoxazole: (see
Hydrochlorothiazide (HCTZ) Alone or in Combination with Triamterene: (see
Dofetilide is eliminated in the kidney by cationic secretion. Inhibitors of renal cationic secretion are contraindicated with dofetilide. In addition, drugs that are actively secreted via this route (e.g., triamterene, metformin, and amiloride) should be co-administered with care as they might increase dofetilide levels.
Dofetilide is metabolized to a small extent by the CYP3A4 isoenzyme of the cytochrome P450 system. Inhibitors of the CYP3A4 isoenzyme could increase systemic dofetilide exposure. Inhibitors of this isoenzyme (e.g., macrolide antibiotics, azole antifungal agents, protease inhibitors, serotonin reuptake inhibitors, amiodarone, cannabinoids, diltiazem, grapefruit juice, nefazadone, norfloxacin, quinine, zafirlukast) should be cautiously co-administered with dofetilide as they can potentially increase dofetilide levels. Dofetilide is not an inhibitor of CYP3A4 nor of other cytochrome P450 isoenzymes (e.g., CYP2C9, CYP2D6) and is not expected to increase levels of drugs metabolized by CYP3A4.
Digoxin: Studies in healthy volunteers have shown that dofetilide does not affect the pharmacokinetics of digoxin. In patients, the concomitant administration of digoxin with dofetilide was associated with a higher occurrence of Torsade de Pointes. It is not clear whether this represents an interaction with dofetilide or the presence of more severe structural heart disease in patients on digoxin; structural heart disease is a known risk factor for arrhythmia. No increase in mortality was observed in patients taking digoxin as concomitant medication.
Other Drugs: In healthy volunteers, amlodipine, phenytoin, glyburide, ranitidine, omeprazole, hormone replacement therapy (a combination of conjugated estrogens and medroxyprogesterone), antacid (aluminum and magnesium hydroxides), and theophylline did not affect the pharmacokinetics of dofetilide. In addition, studies in healthy volunteers have shown that dofetilide does not affect the pharmacokinetics or pharmacodynamics of warfarin, or the pharmacokinetics of propranolol (40 mg twice daily), phenytoin, theophylline, or oral contraceptives.
Population pharmacokinetic analyses were conducted on plasma concentration data from 1445 patients in clinical trials to examine the effects of concomitant medications on clearance or volume of distribution of dofetilide. Concomitant medications were grouped as ACE inhibitors, oral anticoagulants, calcium channel blockers, beta blockers, cardiac glycosides, inducers of CYP3A4, substrates and inhibitors of CYP3A4, substrates and inhibitors of P-glycoprotein, nitrates, sulphonylureas, loop diuretics, potassium sparing diuretics, thiazide diuretics, substrates and inhibitors of tubular organic cation transport, and QTc-prolonging drugs. Differences in clearance between patients on these medications (at any occasion in the study) and those off medications varied between -16% and +3%. The mean clearances of dofetilide were 16% and 15% lower in patients on thiazide diuretics and inhibitors of tubular organic cation transport, respectively.