Doxepin Hydrochloride
Doxepin Hydrochloride Prescribing Information
Doxepin HCl cream, 5% is indicated for the short-term (up to 8 days) management of moderate pruritus in adult patients with atopic dermatitis or lichen simplex chronicus (see
A thin film of doxepin HCl cream, 5% should be applied four times each day with at least a 3 to 4 hour interval between applications. There are no data to establish the safety and effectiveness of doxepin HCl cream, 5% when used for greater than 8 days. Chronic use beyond eight days may result in higher systemic levels and should be avoided. Use of doxepin HCl cream, 5% for longer than 8 days may result in an increased likelihood of contact sensitization.
The risk for sedation may increase with greater body surface area application of doxepin HCl cream, 5% (See
Occlusive dressings may increase the absorption of most topical drugs; therefore, occlusive dressings should not be utilized with doxepin HCl cream, 5%.
Because doxepin HCl has an anticholinergic effect and because significant plasma levels of doxepin are detectable after topical doxepin HCl cream application, the use of doxepin HCl cream is contraindicated in patients with untreated narrow angle glaucoma or a tendency to urinary retention.
Doxepin HCl cream is contraindicated in individuals who have shown previous sensitivity to any of its components.
Systemic Adverse Effects
In controlled clinical trials of patients treated with doxepin HCl cream, the most common systemic adverse event reported was drowsiness. Drowsiness occurred in 71 of 330 (22%) of patients treated with doxepin HCl cream compared to 7 of 334 (2%) of patients treated with vehicle cream. Drowsiness resulted in the premature discontinuation of the drug in approximately 5% of patients treated with doxepin HCl cream in controlled clinical trials.
Local Site Adverse Effects
In controlled clinical trials of patients treated with doxepin HCl cream, the most common local site adverse event reported was burning and/or stinging at the site of application. These occurred in 76 of 330 (23%) of patients treated with doxepin HCl cream compared to 54 of 334 (16%) of patients treated with vehicle cream. Most of these reactions were categorized as "mild"; however, approximately 25% of patients who reported burning and/or stinging reported the reaction as "severe". Four patients treated with doxepin HCl cream withdrew from the study because of the burning and/or stinging.
The table below presents the adverse events reported at an incidence of ≥1% in either doxepin HCl cream or vehicle cream treatment groups during the trials:
Adverse Event | Doxepin HCl Cream N=330 | Vehicle N=334 |
Burning /Stinging | 76 (23.0%) | 54 (16.2%) |
Drowsiness | 71 (21.5%) | 7 (2.1%) |
Dry Mouth1 | 32 (9.7%) | 4 (1.2%) |
Pruritus2 | 13 (3.9%) | 20 (6.0%) |
Fatigue/Tiredness | 10 (3.0%) | 5 (1.5%) |
Exacerbated Eczema | 10 (3.0%) | 8 (2.4%) |
Other Application Site Reaction3 | 10 (3.0%) | 16 (4.8%) |
Dizziness4 | 7 (2.1%) | 3 (0.9%) |
Mental Emotional Changes | 6 (1.8%) | 1 (0.3%) |
Taste Perversion5 | 5 (1.5%) | 1 (0.3%) |
Edema | 4 (1.2%) | 1 (0.3%) |
Headache | 3 (0.9%) | 14 (4.2%) |
1 Includes reports of “dry lips”, “dry throat”, and “thirst” | ||
2 Includes reports of “Pruritus Exacerbated” | ||
3 Includes report of “increased irritation at application site” | ||
4 Includes reports of “lightheadedness” and “dizziness/vertigo” | ||
5 Includes reports of “bitter taste” and “metallic taste in mouth” | ||
Adverse events occurring in 0.5% to < 1.0% of doxepin HCl cream treated patients in the controlled clinical trials included: nervousness/anxiety, tongue numbness, fever, and nausea.
Twenty-six cases of allergic contact dermatitis have been reported in patients using doxepin HCl cream, twenty of which were documented by positive patch test to doxepin 5% cream.
Studies have not been performed examining drug interactions with doxepin HCl cream. However, since plasma levels of doxepin following topical application of doxepin HCl cream can reach levels obtained with oral doxepin HCl therapy, the following drug interactions are possible following topical doxepin HCl cream application:
Drugs Metabolized by P450 2D6
The biochemical activity of the drug metabolizing isozyme cytochrome P450 2D6 (debrisoquin hydroxylase) is reduced in a subset of the Caucasian population (about 7% to 10% of Caucasians are so-called "poor metabolizers"); reliable estimates of the prevalence of reduced P450 2D6 isozyme activity among Asian, African and other populations are not yet available. Poor metabolizers have higher than expected plasma concentrations of tricyclic antidepressants (TCAs) when given usual doses. Depending on the fraction of drug metabolized by P450 2D6, the increase in plasma concentration may be small, or quite large (8-fold increase in plasma AUC of the TCA).
In addition, certain drugs inhibit the activity of this isozyme and make normal metabolizers resemble poor metabolizers. An individual who is stable on a given dosage regimen of a TCA may become abruptly toxic when given one of these inhibiting drugs as concomitant therapy. The drugs that inhibit cytochrome P450 2D6 include some that are not metabolized by the enzyme (quinidine; cimetidine) and many that are substrates for P450 2D6 (many other antidepressants, phenothiazines, and the Type 1C antiarrhythmics propafenone and flecainide). While all the selective serotonin reuptake inhibitors (SSRIs), e.g., fluoxetine, sertraline, and paroxetine, inhibit P450 2D6, they may vary in the extent of inhibition. The extent to which SSRI-TCA interactions may pose clinical problems will depend on the degree of inhibition and the pharmacokinetics of the SSRI involved. Nevertheless, caution is indicated in the co-administration of TCAs with any of the SSRIs. Of particular importance, sufficient time must elapse before initiating TCA treatment in a patient being withdrawn from fluoxetine, given the long half-life of the parent and active metabolite (at least 5 weeks may be necessary).
Concomitant use of tricyclic antidepressants with drugs that can inhibit cytochrome P450 2D6 may require lower doses than usually prescribed for either the tricyclic antidepressant or the other drug. It is desirable to monitor TCA plasma levels whenever a TCA is going to be co-administered with another drug known to be an inhibitor of P450 2D6.
MAO Inhibitors
Serious side effects and even death have been reported following the concomitant use of certain drugs with MAO inhibitors. Therefore, MAO inhibitors should be discontinued at least two weeks prior to the cautious initiation of therapy with doxepin HCl cream. The exact length of time may vary and is dependent upon the particular MAO inhibitor being used, the length of time it has been administered, and the dosage involved.
Cimetidine
Serious anticholinergic symptoms (i.e., severe dry mouth, urinary retention and blurred vision) have been associated with elevations in the serum levels of tricyclic antidepressant when cimetidine therapy is initiated. Additionally, higher than expected tricyclic antidepressant levels have been observed when they are begun in patients already taking cimetidine.
Alcohol
Alcohol ingestion may exacerbate the potential sedative effects of doxepin HCl cream. This is especially important in patients who may use alcohol excessively.
Tolazamide
A case of severe hypoglycemia has been reported in a type II diabetic patient maintained on tolazamide (1 gm/day) 11 days after the addition of oral doxepin (75 mg/day).
Doxepin hydrochloride (HCl) cream, 5% is a topical cream. Each gram contains: 50 mg of doxepin hydrochloride, USP (equivalent to 44.3 mg of doxepin).
Doxepin hydrochloride, USP is one of a class of agents known as dibenzoxepin tricyclic antidepressant compounds. It is an isomeric mixture of N,N-dimethyldibenz[
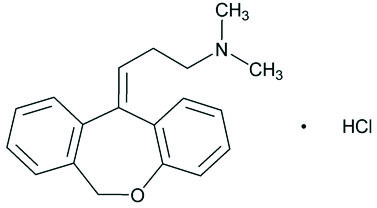
Doxepin HCl cream, 5% also contains sorbitol solution, cetyl alcohol, isopropyl myristate, glyceryl monostearate, PEG-100 stearate, white petrolatum, benzyl alcohol, titanium dioxide and purified water.