Doxorubicin Hydrochloride Prescribing Information
-
Doxorubicin hydrochloride can cause myocardial damage, including acute left ventricular failure. The risk of cardiomyopathy with doxorubicin hydrochloride is generally proportional to the cumulative exposure. Include prior use of other anthracyclines or anthracenediones in calculations of cumulative dose. The risk of cardiomyopathy may be increased at lower cumulative doses in patients with prior mediastinal irradiation.
In a clinical study in 250 patients with advanced cancer who were treated with doxorubicin hydrochloride liposome injection, the risk of cardiomyopathy was 11% when the cumulative anthracycline dose was between 450 mg/m2to 550 mg/m2. Cardiomyopathy was defined as >20% decrease in resting left ventricular ejection fraction (LVEF) from baseline where LVEF remained in the normal range or a >10% decrease in LVEF from baseline where LVEF was less than the institutional lower limit of normal. Two percent of patients developed signs and symptoms of congestive heart failure without documented evidence of cardiomyopathy.
Assess left ventricular cardiac function (e.g. MUGA or echocardiogram) prior to initiation of doxorubicin hydrochloride liposome injection, during treatment to detect acute changes, and after treatment to detect delayed cardiomyopathy. Administer doxorubicin hydrochloride liposome injection to patients with a history of cardiovascular disease only when the potential benefit of treatment outweighs the risk.
-
Serious, life-threatening, and fatal infusion-related reactions characterized by one or more of the following symptoms can occur with doxorubicin hydrochloride liposome injection: flushing, shortness of breath, facial swelling, headache, chills, chest pain, back pain, tightness in the chest and throat, fever, tachycardia, pruritus, rash, cyanosis, syncope, bronchospasm, asthma, apnea, and hypotension. Of 239 patients with ovarian cancer treated with doxorubicin hydrochloride liposome injection in Trial 4, 7% of patients experienced acute infusion-related reactions resulting in dose interruption. All occurred during cycle 1 and none during subsequent cycles. Across multiple studies of doxorubicin hydrochloride liposome injection monotherapy including this and other studies enrolling 760 patients with various solid tumors, 11% of patients had infusion-related reactions. The majority of infusion-related events occurred during the first infusion.
Ensure that medications to treat infusion-related reactions and cardiopulmonary resuscitative equipment are available for immediate use prior to initiation of doxorubicin hydrochloride liposome injection. Initiate doxorubicin hydrochloride liposome injection infusions at a rate of 1 mg/min and increase rate as tolerated [see Dosage and Administration ]. Withhold doxorubicin hydrochloride liposome injection for Grade 1, 2, or 3 infusion-related reactions and resume at a reduced infusion rate. Discontinue doxorubicin hydrochloride liposome injection infusion for serious or life-threatening infusion-related reactions.
Doxorubicin hydrochloride liposome injection: 20 mg/10 mL (2 mg/mL) and 50 mg/25 mL (2 mg/mL) in single-dose vials. The drug product appears as a translucent, red liposomal dispersion.
Doxorubicin hydrochloride liposome injection is contraindicated in patients who have a history of severe hypersensitivity reactions, including anaphylaxis, to doxorubicin hydrochloride [see Warnings and Precautions (
Serious, life-threatening, and fatal infusion-related reactions characterized by one or more of the following symptoms can occur with doxorubicin hydrochloride liposome injection: flushing, shortness of breath, facial swelling, headache, chills, chest pain, back pain, tightness in the chest and throat, fever, tachycardia, pruritus, rash, cyanosis, syncope, bronchospasm, asthma, apnea, and hypotension. Of 239 patients with ovarian cancer treated with doxorubicin hydrochloride liposome injection in Trial 4, 7% of patients experienced acute infusion-related reactions resulting in dose interruption. All occurred during cycle 1 and none during subsequent cycles. Across multiple studies of doxorubicin hydrochloride liposome injection monotherapy including this and other studies enrolling 760 patients with various solid tumors, 11% of patients had infusion-related reactions. The majority of infusion-related events occurred during the first infusion.
Ensure that medications to treat infusion-related reactions and cardiopulmonary resuscitative equipment are available for immediate use prior to initiation of doxorubicin hydrochloride liposome injection. Initiate doxorubicin hydrochloride liposome injection infusions at a rate of 1 mg/min and increase rate as tolerated [see Dosage and Administration ]. Withhold doxorubicin hydrochloride liposome injection for Grade 1, 2, or 3 infusion-related reactions and resume at a reduced infusion rate. Discontinue doxorubicin hydrochloride liposome injection infusion for serious or life-threatening infusion-related reactions.
The following adverse reactions are discussed in more detail in other sections of the labeling.
• Cardiomyopathy [see Warnings and Precautions (
Doxorubicin hydrochloride can cause myocardial damage, including acute left ventricular failure. The risk of cardiomyopathy with doxorubicin hydrochloride is generally proportional to the cumulative exposure. Include prior use of other anthracyclines or anthracenediones in calculations of cumulative dose. The risk of cardiomyopathy may be increased at lower cumulative doses in patients with prior mediastinal irradiation.
In a clinical study in 250 patients with advanced cancer who were treated with doxorubicin hydrochloride liposome injection, the risk of cardiomyopathy was 11% when the cumulative anthracycline dose was between 450 mg/m2to 550 mg/m2. Cardiomyopathy was defined as >20% decrease in resting left ventricular ejection fraction (LVEF) from baseline where LVEF remained in the normal range or a >10% decrease in LVEF from baseline where LVEF was less than the institutional lower limit of normal. Two percent of patients developed signs and symptoms of congestive heart failure without documented evidence of cardiomyopathy.
Assess left ventricular cardiac function (e.g. MUGA or echocardiogram) prior to initiation of doxorubicin hydrochloride liposome injection, during treatment to detect acute changes, and after treatment to detect delayed cardiomyopathy. Administer doxorubicin hydrochloride liposome injection to patients with a history of cardiovascular disease only when the potential benefit of treatment outweighs the risk.
• Infusion-Related Reactions [see Warnings and Precautions (
Serious, life-threatening, and fatal infusion-related reactions characterized by one or more of the following symptoms can occur with doxorubicin hydrochloride liposome injection: flushing, shortness of breath, facial swelling, headache, chills, chest pain, back pain, tightness in the chest and throat, fever, tachycardia, pruritus, rash, cyanosis, syncope, bronchospasm, asthma, apnea, and hypotension. Of 239 patients with ovarian cancer treated with doxorubicin hydrochloride liposome injection in Trial 4, 7% of patients experienced acute infusion-related reactions resulting in dose interruption. All occurred during cycle 1 and none during subsequent cycles. Across multiple studies of doxorubicin hydrochloride liposome injection monotherapy including this and other studies enrolling 760 patients with various solid tumors, 11% of patients had infusion-related reactions. The majority of infusion-related events occurred during the first infusion.
Ensure that medications to treat infusion-related reactions and cardiopulmonary resuscitative equipment are available for immediate use prior to initiation of doxorubicin hydrochloride liposome injection. Initiate doxorubicin hydrochloride liposome injection infusions at a rate of 1 mg/min and increase rate as tolerated [see Dosage and Administration ]. Withhold doxorubicin hydrochloride liposome injection for Grade 1, 2, or 3 infusion-related reactions and resume at a reduced infusion rate. Discontinue doxorubicin hydrochloride liposome injection infusion for serious or life-threatening infusion-related reactions.
• Hand-Foot Syndrome [see Warnings and Precautions (
In Trial 4, the incidence of HFS was 51% of patients in the doxorubicin hydrochloride liposome injection arm and 0.9% of patients in the topotecan arm, including 24% Grade 3 or 4 cases of HFS in doxorubicin hydrochloride liposome injection-treated patients and no Grade 3 or 4 cases in topotecan-treated patients. HFS or other skin toxicity required discontinuation of doxorubicin hydrochloride liposome injection in 4.2% of patients.
HFS was generally observed after 2 or 3 cycles of treatment but may occur earlier. Delay doxorubicin hydrochloride liposome injection for the first episode of Grade 2 or greater HFS [see Dosage and Administration ]. Discontinue doxorubicin hydrochloride liposome injection if HFS is severe and debilitating.
• Secondary Oral Neoplasms [see Warnings and Precautions (
Secondary oral cancers, primarily squamous cell carcinoma, have been reported from post-marketing experience in patients with long-term (more than one year) exposure to doxorubicin hydrochloride liposome injection. These malignancies were diagnosed both during treatment with doxorubicin hydrochloride liposome injection and up to 6 years after the last dose. Examine patients at regular intervals for the presence of oral ulceration or with any oral discomfort that may be indicative of secondary oral cancer.
The altered pharmacokinetics and preferential tissue distribution of liposomal doxorubicin that contributes to enhanced skin toxicity and mucositis compared to free doxorubicin may play a role in the development of oral secondary malignancies with long-term use.
The most common adverse reactions (>20%) observed with doxorubicin hydrochloride liposome injection are asthenia, fatigue, fever, nausea, stomatitis, vomiting, diarrhea, constipation, anorexia, hand-foot syndrome, rash and neutropenia, thrombocytopenia and anemia.
No formal drug interaction studies have been conducted with doxorubicin hydrochloride liposome injection.
Doxorubicin hydrochloride liposome injection is doxorubicin hydrochloride, an anthracycline topoisomerase inhibitor, that is encapsulated in PEGYLATED liposomes for intravenous use.
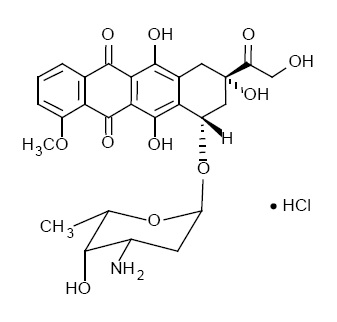
The chemical name of doxorubicin hydrochloride is (8S,10S)-10-[(3-amino-2,3,6-trideoxy-α-L-lyxohexopyranosyl)oxy]-8-glycolyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-nap hthacenedione hydrochloride. The molecular formula is C27H29NO11•HCl; its molecular weight is 579.99.
The structure formula is:
Doxorubicin hydrochloride liposome injection is a sterile, translucent, red liposomal dispersion in 10-mL or 30-mL glass, single-dose vials. Each vial contains 20 mg or 50 mg doxorubicin hydrochloride at a concentration of 2 mg/mL (equivalent to 1.87 mg/mL of doxorubicin) and a pH of 6.5. The PEGYLATED liposome carriers are composed of cholesterol, 3.19 mg/mL; fully hydrogenated soy phosphatidylcholine (HSPC), 9.58 mg/mL; and N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (MPEG-DSPE), 3.19 mg/mL. Each mL also contains ammonium sulfate, approximately 0.6 mg; histidine 1.55 mg as a buffer; hydrochloric acid and/or sodium hydroxide for pH adjustment; and sucrose 94 mg to maintain isotonicity. Greater than 90% of the drug is encapsulated in the PEGYLATED liposomes.
MPEG-DSPE has the following structural formula:
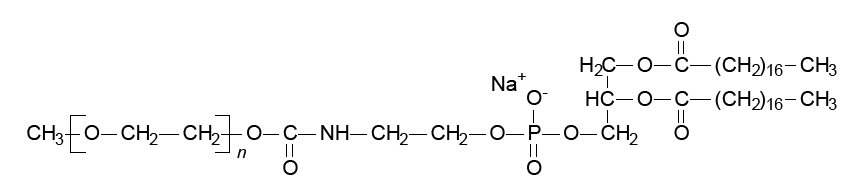
n=ca. 45
HSPC has the following structural formula:
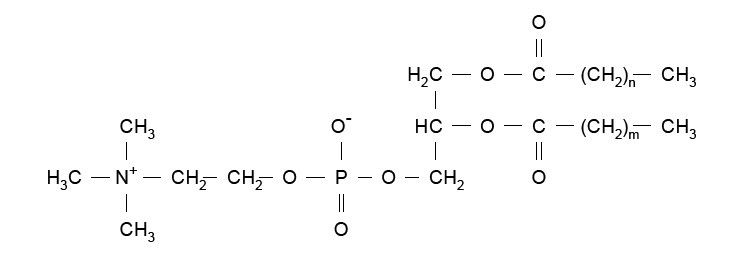
m,n=14 or 16
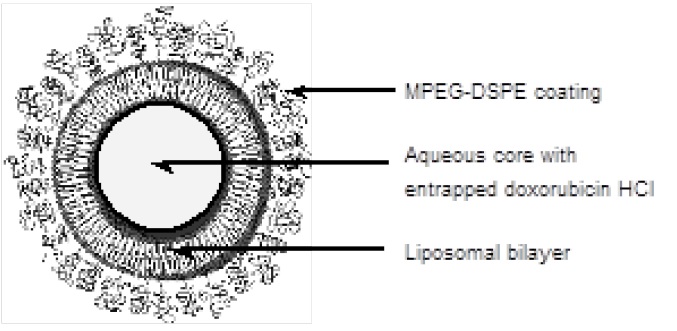
Representation of a PEGYLATED liposome: