Emtricitabine And Tenofovir Disoproxil Fumarate - Emtricitabine And Tenofovir Disoproxil Fumarate tablet, Film Coated
(Emtricitabine And Tenofovir Disoproxil Fumarate)Emtricitabine And Tenofovir Disoproxil Fumarate - Emtricitabine And Tenofovir Disoproxil Fumarate tablet, Film Coated Prescribing Information
Emtricitabine and tenofovir disoproxil fumarate tablets are available in four dose strengths.
- Emtricitabine and Tenofovir Disoproxil Fumarate Tablets, 100 mg/150 mgare supplied as white to off-white, oval-shaped, film-coated tablets, debossed on one side with “AC51” and plain on other side. Each tablet contains 100 mg of emtricitabine (FTC) and 150 mg of tenofovir disoproxil fumarate (TDF) (which is equivalent to 123 mg of tenofovir disoproxil).
- Emtricitabine and Tenofovir Disoproxil Fumarate Tablets, 133 mg/200 mgare supplied as white to off-white, modified capsule shaped, film-coated tablets, debossed on one side with “AC52” and plain on other side. Each tablet contains 133 mg of emtricitabine (FTC) and 200 mg of tenofovir disoproxil fumarate (TDF) (which is equivalent to 163 mg of tenofovir disoproxil).
- Emtricitabine and Tenofovir Disoproxil Fumarate Tablets, 167 mg/250 mgare supplied as white to off-white, modified capsule shaped, film-coated tablets, debossed on one side with “AC53” and plain on other side. Each tablet contains 167 mg of emtricitabine (FTC) and 250 mg of tenofovir disoproxil fumarate (TDF) (which is equivalent to 204 mg of tenofovir disoproxil).
- Emtricitabine and Tenofovir Disoproxil Fumarate Tablets, 200 mg/300 mgare supplied as white to off-white, capsule shaped, film-coated tablets, debossed on one side with “AC24” and plain on other side. Each tablet contains 200 mg of emtricitabine (FTC) and 300 mg of tenofovir disoproxil fumarate (TDF) (which is equivalent to 245 mg of tenofovir disoproxil).
Emtricitabine and tenofovir disoproxil fumarate for HIV-1 PrEP is contraindicated in individuals with unknown or positive HIV-1 status
The following adverse reactions are discussed in other sections of the labeling:
- Severe Acute Exacerbations of Hepatitis B in Patients with HBV Infection [see Warnings and Precautions (5.1)].
- New Onset or Worsening Renal Impairment [see Warnings and Precautions (5.3)].
- Immune Reconstitution Syndrome [see Warnings and Precautions (5.4)].
- Bone Loss and Mineralization Defects [see Warnings and Precautions (5.5)].
- Lactic Acidosis/Severe Hepatomegaly with Steatosis [see Warnings and Precautions (5.6)].
- Tenofovir disoproxil fumarate increases didanosine concentrations. Dose reduction and close monitoring for didanosine toxicity are warranted.
- Co-administration decreases atazanavir concentrations. When co-administered with emtricitabine and tenofovir disoproxil fumarate, use atazanavir given with ritonavir.
- Co-administration of emtricitabine and tenofovir disoproxil fumarate with certain HIV-1 protease inhibitors or certain drugs to treat HCV increases tenofovir concentrations. Monitor for evidence of tenofovir toxicity.
- Consult Full Prescribing Information prior to and during treatment for important drug interactions.
Emtricitabine and tenofovir disoproxil fumarate tablets are fixed-dose combination tablets containing emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF). FTC is a synthetic nucleoside analog of cytidine. TDF is converted
It has a molecular formula of C8H10FN3O3S and a molecular weight of 247.24 g/mol. It has the following structural formula:
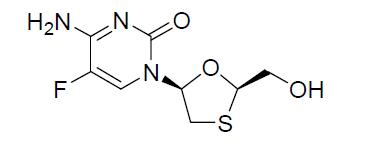
Emtricitabine (FTC) is a white to almost white crystalline powder with a solubility of approximately 112 mg/mL in water at 25ᵒC. The partition coefficient (log p) for emtricitabine is −0.43 and the pKa is 2.65.
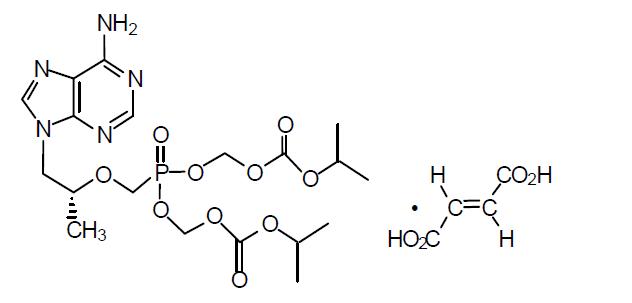
Tenofovir disoproxil fumarate is a white to off-white powder with a solubility of 13.4 mg/mL in water at 25 ᵒC. The partition coefficient (log p) for tenofovir disoproxil is 1.25 and the pKa is 3.75.
All dosages are expressed in terms of TDF except where otherwise noted.
Emtricitabine and tenofovir disoproxil fumarate tablets are for oral administration, and are available in the following strengths:
- Film-coated tablet containing 200 mg of emtricitabine (FTC) and 300 mg of tenofovir disoproxil fumarate (TDF) (which is equivalent to 245 mg of tenofovir disoproxil) as active ingredients.
- Film-coated tablet containing 167 mg of emtricitabine (FTC) and 250 mg of tenofovir disoproxil fumarate (TDF) (which is equivalent to 204 mg of tenofovir disoproxil) as active ingredients.
- Film-coated tablet containing 133 mg of emtricitabine (FTC) and 200 mg of tenofovir disoproxil fumarate (TDF) (which is equivalent to 163 mg of tenofovir disoproxil) as active ingredients.
- Film-coated tablet containing 100 mg of emtricitabine (FTC) and 150 mg of tenofovir disoproxil fumarate (TDF) (which is equivalent to 123 mg of tenofovir disoproxil) as active ingredients.
Emtricitabine and tenofovir disoproxil fumarate tablets also include the following inactive ingredients: calcium stearate, croscarmellose sodium, hypromellose, microcrystalline cellulose, pregelatinized starch, titanium dioxide and triacetin.