Emtriva Prescribing Information
| Dosage and Administration | |
Testing Prior to Initiation of Treatment with EMTRIVA (Prior to or when initiating EMTRIVA, test patients for hepatitis B virus infection [see Warnings and Precautions (5.1)]. | 12/2018 |
| Warnings and Precautions | |
Severe Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV (All patients should be tested for the presence of chronic Hepatitis B virus (HBV) before or when initiating EMTRIVA [see Dosage and Administration (2.1)] .Severe acute exacerbations of hepatitis B (e.g., liver decompensation and liver failure) have been reported in patients who are coinfected with HIV-1 and HBV and have discontinued EMTRIVA. Patients who are coinfected with HIV-1 and HBV who discontinue EMTRIVA should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment. If appropriate, initiation of anti-hepatitis B therapy may be warranted, especially in patients with advanced liver disease or cirrhosis, since posttreatment exacerbation of hepatitis may lead to hepatic decompensation and liver failure. | 12/2018 |
| Coadministration with Related Products | Removed 12/2018 |
EMTRIVA® is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection.
- Testing: Prior to or when initiating EMTRIVA test for hepatitis B virus infection. ()
2.1 Testing Prior to Initiation of Treatment with EMTRIVAPrior to or when initiating EMTRIVA, test patients for hepatitis B virus infection[see Warnings and Precautions (5.1)]. - EMTRIVA may be taken without regard to food. ()
2.2 Recommended DosageEMTRIVA is taken by mouth once daily and may be taken without regard to food
[see Clinical Pharmacology (12.3)]. - Adult Patients (18 years of age and older) ():
2.3 Recommended Dosage in Adult Patients (18 years of age and older)EMTRIVA capsules: One 200 mg capsule administered once daily orally.
EMTRIVA oral solution: 240 mg (24 mL) administered once daily orally.
- EMTRIVA capsules: One 200 mg capsule administered once daily orally.
- EMTRIVA oral solution: 240 mg (24 mL) administered once daily orally.
- Pediatric Patients (0–3 months of age) ():
2.4 Recommended Dosage in Pediatric Patients (0–3 months of age)EMTRIVA oral solution: 3 mg per kg administered once daily orally.
- EMTRIVA oral solution: 3 mg/kg administered once daily orally.
- Pediatric Patients (3 months through 17 years of age) ():
2.5 Recommended Dosage in Pediatric Patients (3 months through 17 years of age)EMTRIVA oral solution: 6 mg per kg up to a maximum of 240 mg (24 mL) administered once daily orally.
EMTRIVA capsules: For pediatric patients weighing more than 33 kg who can swallow an intact capsule, one 200 mg capsule administered once daily orally.
- EMTRIVA capsules: For children weighing more than 33 kg who can swallow an intact capsule, one 200 mg capsule administered once daily orally.
- EMTRIVA oral solution: 6 mg/kg up to a maximum of 240 mg (24 mL) administered once daily orally.
- Dose interval adjustment in adult patients with renal impairment ():
2.6 Dosage Adjustment in Patients with Renal ImpairmentTable 1 provides dosage interval adjustment for patients with renal impairment. No dosage adjustment is necessary for patients with mild renal impairment (creatinine clearance 50–80 mL/min). The safety and effectiveness of dose adjustment recommendations in patients with moderate to severe renal impairment (creatinine clearance below 50 mL/min) have not been clinically evaluated. Therefore, clinical response to treatment and renal function should be closely monitored in these patients
[see Warnings and Precautions (5.4), Use in Specific Populations (8.6)].Table 1 Dose Interval Adjustment for Adult Patients with Altered Creatinine Clearance Creatinine Clearance (mL/min) Formulation ≥50 mL/min 30–49 mL/min 15–29 mL/min <15 mL/min or on hemodialysisHemodialysis Patients: If dosing on day of dialysis, give dose after dialysis. Capsule
(200 mg)200 mg every 24 hours 200 mg every 48 hours 200 mg every 72 hours 200 mg every 96 hours Oral Solution
(10 mg/mL)240 mg every 24 hours
(24 mL)120 mg every 24 hours
(12 mL)80 mg every 24 hours
(8 mL)60 mg every 24 hours
(6 mL)There are insufficient data available to make dosage recommendations in pediatric patients with renal impairment.
| Creatinine Clearance (mL/min) | ||||
|---|---|---|---|---|
| Formulation | ≥50 mL/min | 30–49 mL/min | 15–29 mL/min | <15 mL/min or on hemodialysisHemodialysis Patients: If dosing on day of dialysis, give dose after dialysis. |
| Capsule (200 mg) | 200 mg every 24 hours | 200 mg every 48 hours | 200 mg every 72 hours | 200 mg every 96 hours |
| Oral Solution (10 mg/mL) | 240 mg every 24 hours (24 mL) | 120 mg every 24 hours (12 mL) | 80 mg every 24 hours (8 mL) | 60 mg every 24 hours (6 mL) |
EMTRIVA is available as capsules or as an oral solution.
- 200 mg Capsules: 200 mg of emtricitabine (FTC): size 1 hard gelatin capsules with a blue cap and white body, printed with "200 mg" in black on the cap and with "GILEAD" and the corporate logo in black on the body.
- Oral solution: clear, orange to dark orange liquid containing 10 mg of FTC per mL.
- Lactation: Breastfeeding is not recommended. ()
8.2 LactationRisk SummaryThe Centers for Disease Control and Prevention recommend that HIV-1 infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV-1.
Based on published data, FTC has been shown to be present in human breast milk. It is not known if FTC affects milk production or has effects on the breastfed child.
Because of the potential for: (1) HIV transmission (in HIV-negative infants); (2) developing viral resistance (in HIV-positive infants); and (3) adverse reactions in a breastfed infant similar to those seen in adults, instruct mothers not to breastfeed if they are taking EMTRIVA.
- Pediatrics: Dose adjustment based on age and weight. (,
2.4 Recommended Dosage in Pediatric Patients (0–3 months of age)EMTRIVA oral solution: 3 mg per kg administered once daily orally.
,2.5 Recommended Dosage in Pediatric Patients (3 months through 17 years of age)EMTRIVA oral solution: 6 mg per kg up to a maximum of 240 mg (24 mL) administered once daily orally.
EMTRIVA capsules: For pediatric patients weighing more than 33 kg who can swallow an intact capsule, one 200 mg capsule administered once daily orally.
)12.3 PharmacokineticsAdultsThe pharmacokinetic properties of FTC were evaluated in healthy subjects and HIV-1-infected subjects. Emtricitabine pharmacokinetics are similar between these populations.
Figure 1 shows the mean steady-state plasma FTC concentration-time profile in 20 HIV-1-infected subjects receiving EMTRIVA capsules.
Figure 1 Mean (± 95% CI) Steady-State Plasma FTC Concentrations in HIV-1 Infected Adults (N=20)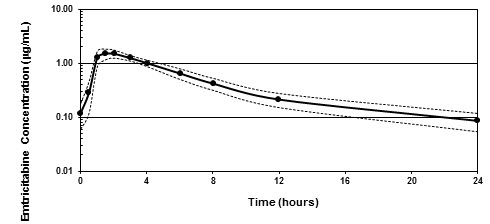

Figure 1 AbsorptionEmtricitabine is rapidly and extensively absorbed following oral administration, with peak plasma concentrations occurring at 1–2 hours postdose. Following multiple dose oral administration of EMTRIVA capsules to 20 HIV-1 infected subjects, the (mean ± SD) steady-state plasma FTC peak concentration (Cmax) was 1.8 ± 0.7 µg/mL and the area-under the plasma concentration-time curve over a 24-hour dosing interval (AUC) was 10.0 ± 3.1 µg
∙hr/mL. The mean steady-state plasma trough concentration at 24 hours postdose was 0.09 µg/mL. The mean absolute bioavailability of EMTRIVA capsules was 93%, while the mean absolute bioavailability of EMTRIVA oral solution was 75%. The relative bioavailability of EMTRIVA oral solution was approximately 80% of EMTRIVA capsules.The multiple dose pharmacokinetics of FTC are dose proportional over a dose range of 25–200 mg.
DistributionIn vitro binding of FTC to human plasma proteins was less than 4% and independent of concentration over the range of 0.02–200 µg/mL. At peak plasma concentration, the mean plasma to blood drug concentration ratio was ~1.0 and the mean semen to plasma drug concentration ratio was ~4.0.
MetabolismFollowing administration of radiolabelled FTC, complete recovery of the dose was achieved in urine (~86%) and feces (~14%). Thirteen percent (13%) of the dose was recovered in urine as three putative metabolites. The biotransformation of FTC includes oxidation of the thiol moiety to form the 3'-sulfoxide diastereomers (~9% of dose) and conjugation with glucuronic acid to form 2'-O-glucuronide (~4% of dose). No other metabolites were identifiable.
EliminationThe plasma FTC half-life is approximately 10 hours. The renal clearance of FTC is greater than the estimated creatinine clearance, suggesting elimination by both glomerular filtration and active tubular secretion. There may be competition for elimination with other compounds that are also renally eliminated.
Effects of Food on Oral AbsorptionEMTRIVA capsules and oral solution may be taken with or without food. Emtricitabine systemic exposure (AUC) was unaffected while Cmaxdecreased by 29% when EMTRIVA capsules were administered with food (an approximately 1000 kcal high-fat meal). Emtricitabine systemic exposure (AUC) and Cmaxwere unaffected when 200 mg EMTRIVA oral solution was administered with either a high-fat or low-fat meal.
Specific PopulationsGeriatric PatientsThe pharmacokinetics of FTC have not been fully evaluated in the elderly (65 years of age and older).
Pediatric PatientsThe pharmacokinetics of FTC at steady state were determined in 77 HIV-1 infected pediatric subjects, and the pharmacokinetic profile was characterized in four age groups (Table 6). The FTC exposure achieved in pediatric subjects receiving a daily dose of 6 mg/kg up to a maximum of 240 mg oral solution or a 200-mg capsule is similar to exposures achieved in adult subjects receiving a once-daily dose of 200 mg.
The pharmacokinetics of FTC were studied in 20 neonates born to HIV-1 positive mothers. Each mother received prenatal and intrapartum combination antiretroviral therapy. Neonates received up to 6 weeks of AZT prophylactically after birth. The neonates were administered two short courses of FTC oral solution (each 3 mg/kg once daily × 4 days) during the first 3 months of life. The AUC observed in neonates who received a daily dose of 3 mg/kg of FTC was similar to the AUC observed in pediatric subjects aged 3 months to 17 years who received a daily dose of FTC as a 6 mg/kg oral solution up to 240 mg or as a 200-mg capsule (Table 6).
Table 6 Mean ± SD Pharmacokinetic Parameters by Age Groups for Pediatric Subjects and Neonates Receiving EMTRIVA Capsules or Oral Solution HIV-1-exposed Neonates HIV-1 Infected Pediatric Subjects Age 0–3 mo
(N=20)Two pharmacokinetic evaluations were conducted in 20 neonates over the first 3 months of life. Median (range) age of infant on day of pharmacokinetic evaluation was 26 (5–81) days.3–24 mo
(N=14)25 mo–6 yr
(N=19)7–12yr
(N=17)13–17 yr
(N=27)Formulation Capsule (n) 0 0 0 10 26 Oral Solution (n) 20 14 19 7 1 Dose (mg/kg)Mean (range). 3.1 (2.9–3.4) 6.1 (5.5–6.8) 6.1 (5.6–6.7) 5.6 (3.1–6.6) 4.4 (1.8–7.0) Cmax(µg/mL) 1.6 ± 0.6 1.9 ± 0.6 1.9 ± 0.7 2.7 ± 0.8 2.7 ± 0.9 AUC (µg ∙hr/mL)11.0 ± 4.2 8.7 ± 3.2 9.0 ± 3.0 12.6 ± 3.5 12.6 ± 5.4 T1/2(hr) 12.1 ± 3.1 8.9 ± 3.2 11.3 ± 6.4 8.2 ± 3.2 8.9 ± 3.3 GenderFTC pharmacokinetics are similar in adult male and female subjects.
RaceNo pharmacokinetic differences due to race have been identified.
Patients with Renal ImpairmentThe pharmacokinetics of FTC are altered in subjects with renal impairment
[see Warnings and Precautions (5.4)]. In adult subjects with creatinine clearance below 50 mL/min or with end-stage renal disease (ESRD) requiring dialysis, Cmaxand AUC of FTC were increased (Table 7). The effects of renal impairment on FTC pharmacokinetics in pediatric patients are not known.Table 7 Pharmacokinetic Parameters (Mean ± SD) of FTC in Adult Subjects with Varying Degrees of Renal Function Creatinine Clearance (mL/min) >80
(N=6)50–80
(N=6)30–49
(N=6)<30
(N=5)ESRDESRD subjects requiring dialysis
<30
(N=5)Baseline creatinine clearance (mL/min) 107 ± 21 59.8 ± 6.5 40.9 ± 5.1 22.9 ± 5.3 8.8 ± 1.4 Cmax(µg/mL) 2.2 ± 0.6 3.8 ± 0.9 3.2 ± 0.6 2.8 ± 0.7 2.8 ± 0.5 AUC (µg ∙hr/mL)11.8 ± 2.9 19.9 ± 1.2 25.1 ± 5.7 33.7± 2.1 53.2 ± 9.9 CL/F (mL/min) 302 ± 94 168 ± 10 138 ± 28 99 ± 6 64 ± 12 CLr (mL/min) 213 ± 89 121 ± 39 69 ± 32 30 ± 11 NANA = Not Applicable Patients with Hepatic ImpairmentThe pharmacokinetics of FTC have not been studied in subjects with hepatic impairment; however, FTC is not significantly metabolized by liver enzymes, so the impact of liver impairment should be limited.
Assessment of Drug InteractionsAt concentrations up to 14-fold higher than those observed in vivo, FTC did not inhibit in vitro drug metabolism mediated by any of the following human CYP isoforms: CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. FTC did not inhibit the enzyme responsible for glucuronidation (uridine-5'-disphosphoglucuronyl transferase). Based on the results of these in vitro experiments and the known elimination pathways of FTC, the potential for CYP-mediated interactions involving FTC with other medicinal products is low.
EMTRIVA has been evaluated in healthy volunteers in combination with TDF, AZT, indinavir, famciclovir, and d4T. Tables 8 and 9 summarize the pharmacokinetic effects of coadministered drug on FTC pharmacokinetics and effects of FTC on the pharmacokinetics of coadministered drug.
Table 8 Drug Interactions: Change in Pharmacokinetic Parameters for FTC in the Presence of the Coadministered DrugAll interaction trials conducted in healthy volunteers. Coadministered Drug Dose of Coadministered Drug (mg) FTC Dose (mg) N % Change of FTC Pharmacokinetic Parameters↑ = Increase; ⇔ = No Effect; NA = Not Applicable(90% CI) Cmax AUC Cmin Famciclovir 500 × 1 200 × 1 12 ⇔ ⇔ NA Indinavir 800 × 1 200 × 1 12 ⇔ ⇔ NA Stavudine 40 × 1 200 × 1 6 ⇔ ⇔ NA Tenofovir DF 300 once daily × 7 days 200 once daily × 7 days 17 ⇔ ⇔ ↑ 20
(↑ 12 to ↑ 29)Zidovudine 300 twice daily × 7 days 200 once daily × 7 days 27 ⇔ ⇔ ⇔ Table 9 Drug Interactions: Change in Pharmacokinetic Parameters for Coadministered Drug in the Presence of FTCAll interaction trials conducted in healthy volunteers. Coadministered Drug Dose of Coadministered Drug (mg) FTC Dose (mg) N % Change of Coadministered Drug Pharmacokinetic Parameters↑ = Increase; ⇔ = No Effect; NA = Not Applicable(90% CI) Cmax AUC Cmin Famciclovir 500 × 1 200 × 1 12 ⇔ ⇔ NA Indinavir 800 × 1 200 × 1 12 ⇔ ⇔ NA Stavudine 40 × 1 200 × 1 6 ⇔ ⇔ NA Tenofovir DF 300 once daily × 7 days 200 once daily × 7 days 17 ⇔ ⇔ ⇔ Zidovudine 300 twice daily × 7 days 200 once daily × 7 days 27 ↑ 17
(↑ 0 to ↑ 38)↑ 13
(↑ 5 to↑ 20)⇔