Encelto
(Revakinagene Taroretcel-Lwey)Encelto Prescribing Information
ENCELTO is indicated for the treatment of adults with idiopathic macular telangiectasia type 2 (MacTel).
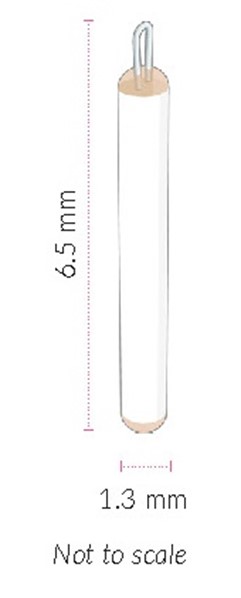
ENCELTO is a single-dose implant that contains 200,000 to 440,000 allogeneic retinal pigment epithelial cells expressing recombinant human ciliary neurotrophic factor (rhCNTF) (NTC-201-6A cell line) for intravitreal surgical placement. ENCELTO is an opaque semi-permeable capsule that is white to off-white, capped on both ends, and has a titanium loop on one end. The ENCELTO width is 1.2 ± 0.1 mm, its length is 6.1 ± 0.4 mm, and its internal diameter is 0.88 ± 0.02 mm (
ENCELTO is contraindicated in patients with:
- Active or suspected ocular or periocular infections.
- Known hypersensitivity to Endothelial Serum Free Media (Endo-SFM)
ENCELTO (revakinagene taroretcel-lwey) implant, is single-dose, sterile, nonpyrogenic and retrievable.
ENCELTO is an allogeneic encapsulated cell-based gene therapy that contains 200,000 to 440,000 allogeneic retinal pigment epithelial cells expressing recombinant human ciliary neurotrophic factor (rhCNTF) (NTC-201-6A cell line) for surgical intravitreal placement.
ENCELTO consists of an opaque, semi-permeable white to off-white capsule surrounding a scaffold of polyethylene terephthalate (PET) yarn, loaded with rhCNTF secreting allogeneic retinal pigment epithelial cells (NTC-201-6A cell line). Each end of the semi-permeable capsule is sealed with medical grade methacrylate adhesive, and to one end a titanium fixation loop is attached. ENCELTO width is 1.2 ± 0.1 mm, length is 6.1 ± 0.4 mm, and its internal diameter is 0.88 ± 0.02 mm (

ENCELTO is packaged in a protective inner container within an orange to pink liquid hold medium referred to as Endothelial Serum Free Media (Endo-SFM), which is maintained sterile by a sealed outer container. ENCELTO is provided attached, by the fixation loop, to a gripper that both suspends ENCELTO in the Endo-SFM and facilitates intraocular insertion. The Endo-SFM within the packaging inner container may contain visible particles generally described as fiber, solid, white, or metallic in appearance.
ENCELTO is manufactured using animal and human derived reagents.
The efficacy of ENCELTO was evaluated in two studies, Study NTMT-03-A ( NCT03316300; Study 1) and Study NTMT-03-B ( NCT03319849; Study 2) as described below.
Study 1 was a randomized, multi-center, sham-controlled study which enrolled adults with MacTel. For enrollment, the patients were required to have a photoreceptor inner segment/outer segment (IS/OS PR) break (loss) in ellipsoid zone (EZ) between 0.16 and 2.00 mm2 measured by spectral domain-optical coherence tomography (SD-OCT) and best corrected visual acuity (BCVA) of 54-letter score or better (20/80 or better) as measured by the Early Treatment Diabetic Retinopathy Study (ETDRS) chart at screening. Patients with neovascular MacTel were excluded. Patients were randomized to receive either ENCELTO intravitreal implant or sham procedure under standard operative procedures. Patients in ENCELTO group underwent conjunctival peritomy, implant placement in the vitreous cavity via sclerotomy and closure with sutures. Patients in the Sham group underwent conjunctival peritomy, scleral pressure, and conjunctival closure with sutures. One hundred and fifteen (96%) of 120 patients underwent the assigned procedure and were included in the analysis of efficacy.
A total of 120 patients were randomized and of these, 115 patients (ENCELTO group: 58; Sham group: 57) comprise the efficacy analysis population. The demographic characteristics of the efficacy analysis population were as follows: the mean age was 61 years (range 40 to 78 years), 79 patients (69%) were female, 98 patients (85%) were White, 5 patients (4%) were Asian, 3 patients (3%) were Black or African American, 1 patient (1%) was American Indian, and 8 patients (7%) were of “other” race. Six patients (5%) were Hispanic. The median (min, max) baseline EZ area loss was 0.35 (0.15, 1.99) mm2 for the ENCELTO group and 0.36 (0.16, 1.7) mm2 for the Sham group. The median (min, max) baseline aggregate sensitivity of microperimetry within the EZ break area 35.2 (0.75, 398.8) dB for the ENCELTO group and 35.5 (2, 281.3) dB for the Sham group.
The primary efficacy outcome measure was the rate of change in the area of EZ loss (IS/OS, macular PR loss) over 24 months, as measured by SD-OCT. The secondary outcome measure was the mean change in aggregate sensitivity loss of microperimetry within the EZ break area from baseline to Month 24.
The efficacy outcome results for Study 1 are summarized in
Efficacy endpoints | ENCELTO n= 58 | Sham n=57 | Difference ENCELTO-Sham | P-valuec |
Rate of change in EZ area loss from baseline over 24 monthsa mm2 (95% CI) | 0.075 (0.05, 0.10) | 0.166 (0.14, 0.19) | -0.091 (-0.13, -0.06) | <0.0001 |
Mean change in aggregate retinal sensitivity loss from baseline to 24-monthsb dB (95% CI) | 25.27 (15.88, 34.67) | 43.02 (31.78, 54.26) | -17.75 (-32.58, -2.91) | 0.02 |
Efficacy endpoints | ENCELTO n= 58 | Sham n=57 | Difference ENCELTO-Sham | P-valuec |
Rate of change in EZ area loss from baseline over 24 monthsa mm2 (95% CI) | 0.075 (0.05, 0.10) | 0.166 (0.14, 0.19) | -0.091 (-0.13, -0.06) | <0.0001 |
Mean change in aggregate retinal sensitivity loss from baseline to 24-monthsb dB (95% CI) | 25.27 (15.88, 34.67) | 43.02 (31.78, 54.26) | -17.75 (-32.58, -2.91) | 0.02 |
CI = confidence interval, EZ=ellipsoid zone
a Estimated by using a longitudinal mixed model including EZ area loss as the dependent variable, patient-specific random intercepts, treatment group, time (continuous), and interaction between treatment and time as covariates. The baseline and Months 12, 16, 20, and 24 visits were included.
b Estimated by using two-sample t-test; seven ENCELTO and four Sham patients were excluded due to missing data.
c Statistically significant at two-sided alpha of 0.05.
Study 2 was a randomized, multi-center, sham-controlled study which enrolled adult with MacTel. For enrollment, the patients were required to have an IS/OS PR break in EZ between 0.16 and 2.00 mm2 measured by SD-OCT and BCVA of 54-letter score or better (20/80 or better) as measured by the ETDRS chart at screening. Patients with neovascular MacTel were excluded.
Patients were randomized to receive either ENCELTO intravitreal implant or sham procedure under standard peri-operative procedures. Patients in ENCELTO group underwent conjunctival peritomy, implant placement in the vitreous cavity via sclerotomy and closure with sutures. Patients in the Sham group underwent conjunctival peritomy, scleral pressure, and conjunctival closure with sutures. One hundred and thirteen (95%) of the 119 patients underwent the assigned procedure and were included in efficacy evaluation.
A total of 119 patients were randomized and of these, 113 patients (ENCELTO group: 59; Sham group: 54) comprise the efficacy analysis population. The demographic characteristics of the efficacy analysis population were as follows: the mean age was 59 years (range: 40 to 75 years), 82 patients (73%) were female, 102 patients (90%) were White, 4 patients (4%) were Asian, and 7 patients (6%) were of “other” race or “unable to specify” race. Eight patients (7%) were Hispanic. The median (min, max) baseline EZ area loss was 0.48 (0.16, 1.63) mm2 for the ENCELTO and 0.39 (0.16, 1.38) mm2 for the Sham group. The median (min, max) baseline aggregate sensitivity of microperimetry within the EZ break area 40.07 (4.82, 291.52) dB for the ENCELTO group and 28.86 (0.33, 221.17) dB for the Sham group.
The primary efficacy outcome measure was the rate of change in the area of EZ loss (IS/OS, macular PR loss) over 24 months, as measured by SD-OCT. The secondary outcome measure was the mean change in aggregate sensitivity loss of microperimetry within the EZ break area from baseline to Month 24.
The efficacy results from Study 2 are summarized in
Efficacy endpoints | ENCELTO n= 59 | Sham n=54 | Difference ENCELTO-Sham | P-valuec |
Rate of change in EZ area loss from baseline over 24 monthsa mm2 (95% CI) | 0.111 (0.08, 0.14) | 0.160 (0.13, 0.19) | -0.049 (-0.089, -0.008) | 0.0186c |
Mean change in aggregate retinal sensitivity loss from baseline to 24-monthb dB (95% CI) | 40.02 (26.08, 53.96) | 41.97 (30.34, 53.60) | -1.95 (-20.33, 16.43) | 0.83 |
Efficacy endpoints | ENCELTO n= 59 | Sham n=54 | Difference ENCELTO-Sham | P-valuec |
Rate of change in EZ area loss from baseline over 24 monthsa mm2 (95% CI) | 0.111 (0.08, 0.14) | 0.160 (0.13, 0.19) | -0.049 (-0.089, -0.008) | 0.0186c |
Mean change in aggregate retinal sensitivity loss from baseline to 24-monthb dB (95% CI) | 40.02 (26.08, 53.96) | 41.97 (30.34, 53.60) | -1.95 (-20.33, 16.43) | 0.83 |
CI = confidence interval, EZ=ellipsoid zone
a Estimated by using a longitudinal mixed model including EZ area loss as the dependent variable, patient-specific random intercepts, treatment group, time (continuous), and interaction between treatment and time as covariates. The baseline and Months 12, 16, 20, and 24 visits were included.
b Estimated by using two-sample t-test; Seven ENCELTO and six Sham patients were excluded due to missing data.
c Statistically significant at two-sided alpha of 0.05.