Enilloring Prescribing Information
Warnings and Precautions (5.4) 06/2024
EnilloRing is indicated for use by females of reproductive age to prevent pregnancy.
EnilloRing (etonogestrel/ethinyl estradiol vaginal ring) is a non-biodegradable, flexible, transparent, colorless to almost colorless, combination contraceptive vaginal ring, with an outer diameter of 54 mm and a cross-sectional diameter of 4 mm. It is made of ethylene vinylacetate copolymers and magnesium stearate, and contains 11.7 mg etonogestrel and 2.7 mg ethinyl estradiol. When placed in the vagina, each ring releases on average 0.120 mg/day of etonogestrel and 0.015 mg/day of ethinyl estradiol over a three-week period of use. EnilloRing is not made with natural rubber latex.
- Nursing mothers: Not recommended; can decrease milk production. ()8.2 LactationRisk Summary
Small amounts of contraceptive steroids and/or metabolites, including etonogestrel and ethinyl estradiol are transferred to human milk. Harmful effects have not been observed in breastfed infants exposed to CHCs through breast milk. CHCs can reduce milk production in breastfeeding mothers. This is less likely to occur once breastfeeding is well-established; however, it can occur at any time in some women.When possible, advise the nursing mother to use non-estrogen-containing contraception until she has completely weaned her child. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for EnilloRing and any potential adverse effects on the breastfed child from EnilloRing or from the underlying maternal condition.
EnilloRing is contraindicated in females who are known to have or develop the following conditions:
- A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:
- Smoke, if over age 35[seeand Warnings and Precautions (
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination hormonal contraceptive (CHC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, CHCs, includingEnilloRing, should not be used by women who are over 35 years of age and smoke. [see Contraindications ]WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTSSee full prescribing information for complete boxed warning.- Women over 35 years old who smoke should not use EnilloRing
- Cigarette smoking increases the risk of serious cardiovascular events from combination hormonal contraceptive (CHC) use.
)]5.1 Thromboembolic Disorders and Other Vascular ProblemsStop EnilloRing use if an arterial thrombotic or venous thromboembolic event (VTE) occurs. Stop EnilloRing use if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
[See Adverse Reactions .]If feasible, stop EnilloRing at least four weeks before and through two weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism, and during and following prolonged immobilization.
Start EnilloRing no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
The use of CHCs increases the risk of VTE. Known risk factors for VTE include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of CHCs
[see Contraindications ].Two epidemiologic studies1, 2, 3that assessed the risk of VTE associated with the use of EnilloRing are described below.
In these studies, which were required or sponsored by regulatory agencies, EnilloRing users had a risk of VTE similar to Combined Oral Contraceptives (COCs) users (see Table 1 for adjusted hazard ratios). A large prospective, observational study, the Transatlantic Active Surveillance on Cardiovascular Safety of EnilloRing (TASC), investigated the risk of VTE for new users, and women who were switching to or restarting EnilloRing or COCs in a population that is representative of routine clinical users. The women were followed for 24 to 48 months. The results showed a similar risk of VTE among EnilloRing users (VTE incidence 8.3 per 10,000 WY) and women using COCs (VTE incidence 9.2 per 10,000 WY). For women using COCs that did not contain the progestins desogestrel (DSG) or gestodene (GSD), VTE incidence was 8.9 per 10,000 WY.
A retrospective cohort study using data from 4 health plans in the US (FDA-funded Study in Kaiser Permanente and Medicaid databases) showed the VTE incidence for new users of EnilloRing to be 11.4 events per 10,000 WY, for new users of a levonorgestrel (LNG)-containing COC 9.2 events per 10,000 WY, and for users of other COCs available during the course of the study* 8.2 events per 10,000 WY.
*Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel.
Table 1: Estimates (Hazard Ratios) of Venous Thromboembolism Risk in Users of EnilloRing Compared to Users of Combined Oral Contraceptives (COCs)Epidemiologic Study(Author, Year of Publication) Population StudiedComparator Product(s)Hazard Ratios (HR)(95% CI)TASC (Dinger, 2012) Initiators, including new users, switchers and restarters
All COCs available during the course of the study * COCs available excluding DSG-or GSD -containing OCs
HR†: 0.8 (0.5-1.5)
HR†: 0.8 (0.4-1.7)
FDA-funded Study in Kaiser Permanente and Medicaid databases (Sidney, 2011)
First use of a combined hormonal contraceptive (CHC) during the study period
COCs available during the course of the study‡
LNG/0.03 mg ethinyl estradiol
HR§: 1.1 (0.6-2.2)
HR§: 1.0 (0.5-2.0)
* Includes low-dose COCs containing the following progestins: chlormadinone acetate, cyproterone acetate, desogestrel, dienogest, drospirenone, ethynodiol diacetate, gestodene, levonorgestrel, norethindrone, norgestimate, or norgestrel
† Adjusted for age, BMI, duration of use, VTE history
‡Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel
§Adjusted for age, site, year of entry into studyAn increased risk of thromboembolic and thrombotic disease associated with the use of CHCs is well-established. Although the absolute VTE rates are increased for users of CHCs compared to non-users, the rates associated with pregnancy are even greater, especially during the post-partum period (see Figure 1).
The frequency of VTE in women using CHCs has been estimated to be 3 to 12 cases per 10,000 women-years.
The risk of VTE is highest during the first year of CHC use and after restarting a CHC following a break of at least four weeks. The risk of VTE due to CHCs gradually disappears after use is discontinued.Figure 1 shows the risk of developing a VTE for women who are not pregnant and do not use CHCs, for women who use CHCs, for pregnant women, and for women in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 women who are not pregnant and do not use CHCs are followed for one year, between 1 and 5 of these women will develop a VTE.
Figure 1: Likelihood of Developing a VTE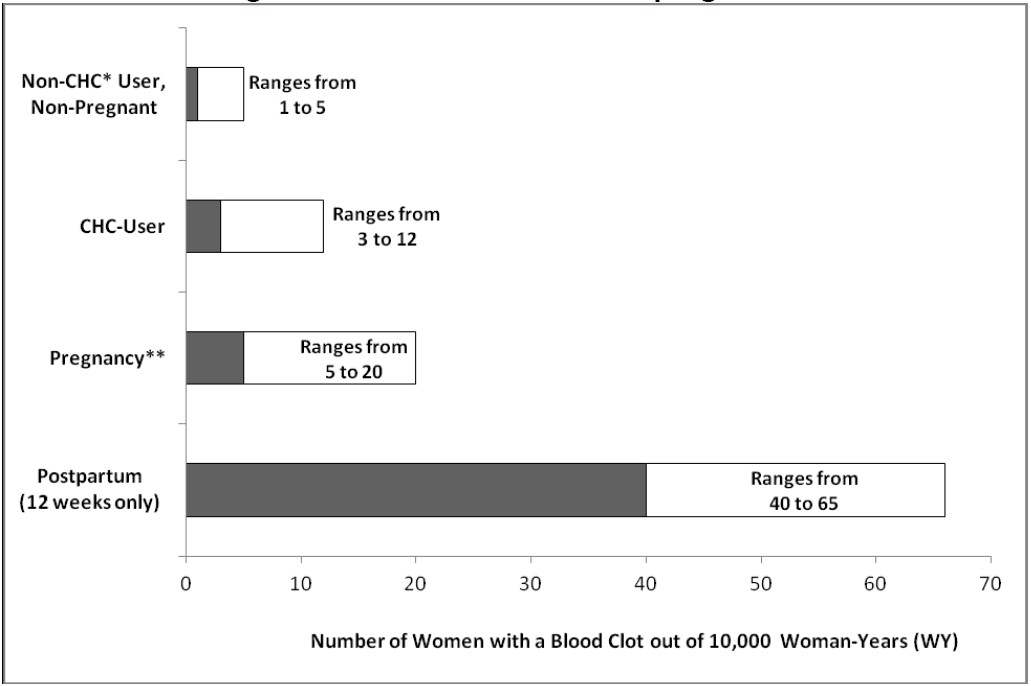
*CHC=combination hormonal contraception
**Pregnancy data based on actual duration of pregnancy in the reference studies. Based on a model assumption that pregnancy duration is nine months, the rate is 7 to 27 per 10,000 WY.Several epidemiology studies indicate that third generation oral contraceptives, including those containing desogestrel (etonogestrel, the progestin in EnilloRing, is the biologically active metabolite of desogestrel), may be associated with a higher risk of VTE than oral contraceptives containing other progestins. Some of these studies indicate an approximate two-fold increased risk. However, data from other studies have not shown this two-fold increase in risk.
Use of CHCs also increases the risk of arterial thromboses such as strokes and myocardial infarctions, especially in women with other risk factors for these events. CHCs have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes). In general, the risk is greatest among older (>35 years of age), hypertensive women who also smoke.
Use EnilloRing with caution in women with cardiovascular disease risk factors.

image description - Have deep vein thrombosis or pulmonary embolism, now or in the past [see Warnings and Precautions ()]5.1 Thromboembolic Disorders and Other Vascular Problems
Stop EnilloRing use if an arterial thrombotic or venous thromboembolic event (VTE) occurs. Stop EnilloRing use if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
[See Adverse Reactions .]If feasible, stop EnilloRing at least four weeks before and through two weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism, and during and following prolonged immobilization.
Start EnilloRing no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
The use of CHCs increases the risk of VTE. Known risk factors for VTE include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of CHCs
[see Contraindications ].Two epidemiologic studies1, 2, 3that assessed the risk of VTE associated with the use of EnilloRing are described below.
In these studies, which were required or sponsored by regulatory agencies, EnilloRing users had a risk of VTE similar to Combined Oral Contraceptives (COCs) users (see Table 1 for adjusted hazard ratios). A large prospective, observational study, the Transatlantic Active Surveillance on Cardiovascular Safety of EnilloRing (TASC), investigated the risk of VTE for new users, and women who were switching to or restarting EnilloRing or COCs in a population that is representative of routine clinical users. The women were followed for 24 to 48 months. The results showed a similar risk of VTE among EnilloRing users (VTE incidence 8.3 per 10,000 WY) and women using COCs (VTE incidence 9.2 per 10,000 WY). For women using COCs that did not contain the progestins desogestrel (DSG) or gestodene (GSD), VTE incidence was 8.9 per 10,000 WY.
A retrospective cohort study using data from 4 health plans in the US (FDA-funded Study in Kaiser Permanente and Medicaid databases) showed the VTE incidence for new users of EnilloRing to be 11.4 events per 10,000 WY, for new users of a levonorgestrel (LNG)-containing COC 9.2 events per 10,000 WY, and for users of other COCs available during the course of the study* 8.2 events per 10,000 WY.
*Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel.
Table 1: Estimates (Hazard Ratios) of Venous Thromboembolism Risk in Users of EnilloRing Compared to Users of Combined Oral Contraceptives (COCs)Epidemiologic Study(Author, Year of Publication) Population StudiedComparator Product(s)Hazard Ratios (HR)(95% CI)TASC (Dinger, 2012) Initiators, including new users, switchers and restarters
All COCs available during the course of the study * COCs available excluding DSG-or GSD -containing OCs
HR†: 0.8 (0.5-1.5)
HR†: 0.8 (0.4-1.7)
FDA-funded Study in Kaiser Permanente and Medicaid databases (Sidney, 2011)
First use of a combined hormonal contraceptive (CHC) during the study period
COCs available during the course of the study‡
LNG/0.03 mg ethinyl estradiol
HR§: 1.1 (0.6-2.2)
HR§: 1.0 (0.5-2.0)
* Includes low-dose COCs containing the following progestins: chlormadinone acetate, cyproterone acetate, desogestrel, dienogest, drospirenone, ethynodiol diacetate, gestodene, levonorgestrel, norethindrone, norgestimate, or norgestrel
† Adjusted for age, BMI, duration of use, VTE history
‡Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel
§Adjusted for age, site, year of entry into studyAn increased risk of thromboembolic and thrombotic disease associated with the use of CHCs is well-established. Although the absolute VTE rates are increased for users of CHCs compared to non-users, the rates associated with pregnancy are even greater, especially during the post-partum period (see Figure 1).
The frequency of VTE in women using CHCs has been estimated to be 3 to 12 cases per 10,000 women-years.
The risk of VTE is highest during the first year of CHC use and after restarting a CHC following a break of at least four weeks. The risk of VTE due to CHCs gradually disappears after use is discontinued.Figure 1 shows the risk of developing a VTE for women who are not pregnant and do not use CHCs, for women who use CHCs, for pregnant women, and for women in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 women who are not pregnant and do not use CHCs are followed for one year, between 1 and 5 of these women will develop a VTE.
Figure 1: Likelihood of Developing a VTE
*CHC=combination hormonal contraception
**Pregnancy data based on actual duration of pregnancy in the reference studies. Based on a model assumption that pregnancy duration is nine months, the rate is 7 to 27 per 10,000 WY.Several epidemiology studies indicate that third generation oral contraceptives, including those containing desogestrel (etonogestrel, the progestin in EnilloRing, is the biologically active metabolite of desogestrel), may be associated with a higher risk of VTE than oral contraceptives containing other progestins. Some of these studies indicate an approximate two-fold increased risk. However, data from other studies have not shown this two-fold increase in risk.
Use of CHCs also increases the risk of arterial thromboses such as strokes and myocardial infarctions, especially in women with other risk factors for these events. CHCs have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes). In general, the risk is greatest among older (>35 years of age), hypertensive women who also smoke.
Use EnilloRing with caution in women with cardiovascular disease risk factors.

image description - Have cerebrovascular disease [see Warnings and Precautions ()]5.1 Thromboembolic Disorders and Other Vascular Problems
Stop EnilloRing use if an arterial thrombotic or venous thromboembolic event (VTE) occurs. Stop EnilloRing use if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
[See Adverse Reactions .]If feasible, stop EnilloRing at least four weeks before and through two weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism, and during and following prolonged immobilization.
Start EnilloRing no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
The use of CHCs increases the risk of VTE. Known risk factors for VTE include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of CHCs
[see Contraindications ].Two epidemiologic studies1, 2, 3that assessed the risk of VTE associated with the use of EnilloRing are described below.
In these studies, which were required or sponsored by regulatory agencies, EnilloRing users had a risk of VTE similar to Combined Oral Contraceptives (COCs) users (see Table 1 for adjusted hazard ratios). A large prospective, observational study, the Transatlantic Active Surveillance on Cardiovascular Safety of EnilloRing (TASC), investigated the risk of VTE for new users, and women who were switching to or restarting EnilloRing or COCs in a population that is representative of routine clinical users. The women were followed for 24 to 48 months. The results showed a similar risk of VTE among EnilloRing users (VTE incidence 8.3 per 10,000 WY) and women using COCs (VTE incidence 9.2 per 10,000 WY). For women using COCs that did not contain the progestins desogestrel (DSG) or gestodene (GSD), VTE incidence was 8.9 per 10,000 WY.
A retrospective cohort study using data from 4 health plans in the US (FDA-funded Study in Kaiser Permanente and Medicaid databases) showed the VTE incidence for new users of EnilloRing to be 11.4 events per 10,000 WY, for new users of a levonorgestrel (LNG)-containing COC 9.2 events per 10,000 WY, and for users of other COCs available during the course of the study* 8.2 events per 10,000 WY.
*Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel.
Table 1: Estimates (Hazard Ratios) of Venous Thromboembolism Risk in Users of EnilloRing Compared to Users of Combined Oral Contraceptives (COCs)Epidemiologic Study(Author, Year of Publication) Population StudiedComparator Product(s)Hazard Ratios (HR)(95% CI)TASC (Dinger, 2012) Initiators, including new users, switchers and restarters
All COCs available during the course of the study * COCs available excluding DSG-or GSD -containing OCs
HR†: 0.8 (0.5-1.5)
HR†: 0.8 (0.4-1.7)
FDA-funded Study in Kaiser Permanente and Medicaid databases (Sidney, 2011)
First use of a combined hormonal contraceptive (CHC) during the study period
COCs available during the course of the study‡
LNG/0.03 mg ethinyl estradiol
HR§: 1.1 (0.6-2.2)
HR§: 1.0 (0.5-2.0)
* Includes low-dose COCs containing the following progestins: chlormadinone acetate, cyproterone acetate, desogestrel, dienogest, drospirenone, ethynodiol diacetate, gestodene, levonorgestrel, norethindrone, norgestimate, or norgestrel
† Adjusted for age, BMI, duration of use, VTE history
‡Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel
§Adjusted for age, site, year of entry into studyAn increased risk of thromboembolic and thrombotic disease associated with the use of CHCs is well-established. Although the absolute VTE rates are increased for users of CHCs compared to non-users, the rates associated with pregnancy are even greater, especially during the post-partum period (see Figure 1).
The frequency of VTE in women using CHCs has been estimated to be 3 to 12 cases per 10,000 women-years.
The risk of VTE is highest during the first year of CHC use and after restarting a CHC following a break of at least four weeks. The risk of VTE due to CHCs gradually disappears after use is discontinued.Figure 1 shows the risk of developing a VTE for women who are not pregnant and do not use CHCs, for women who use CHCs, for pregnant women, and for women in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 women who are not pregnant and do not use CHCs are followed for one year, between 1 and 5 of these women will develop a VTE.
Figure 1: Likelihood of Developing a VTE
*CHC=combination hormonal contraception
**Pregnancy data based on actual duration of pregnancy in the reference studies. Based on a model assumption that pregnancy duration is nine months, the rate is 7 to 27 per 10,000 WY.Several epidemiology studies indicate that third generation oral contraceptives, including those containing desogestrel (etonogestrel, the progestin in EnilloRing, is the biologically active metabolite of desogestrel), may be associated with a higher risk of VTE than oral contraceptives containing other progestins. Some of these studies indicate an approximate two-fold increased risk. However, data from other studies have not shown this two-fold increase in risk.
Use of CHCs also increases the risk of arterial thromboses such as strokes and myocardial infarctions, especially in women with other risk factors for these events. CHCs have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes). In general, the risk is greatest among older (>35 years of age), hypertensive women who also smoke.
Use EnilloRing with caution in women with cardiovascular disease risk factors.

image description - Have coronary artery disease [see Warnings and Precautions ()]5.1 Thromboembolic Disorders and Other Vascular Problems
Stop EnilloRing use if an arterial thrombotic or venous thromboembolic event (VTE) occurs. Stop EnilloRing use if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
[See Adverse Reactions .]If feasible, stop EnilloRing at least four weeks before and through two weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism, and during and following prolonged immobilization.
Start EnilloRing no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
The use of CHCs increases the risk of VTE. Known risk factors for VTE include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of CHCs
[see Contraindications ].Two epidemiologic studies1, 2, 3that assessed the risk of VTE associated with the use of EnilloRing are described below.
In these studies, which were required or sponsored by regulatory agencies, EnilloRing users had a risk of VTE similar to Combined Oral Contraceptives (COCs) users (see Table 1 for adjusted hazard ratios). A large prospective, observational study, the Transatlantic Active Surveillance on Cardiovascular Safety of EnilloRing (TASC), investigated the risk of VTE for new users, and women who were switching to or restarting EnilloRing or COCs in a population that is representative of routine clinical users. The women were followed for 24 to 48 months. The results showed a similar risk of VTE among EnilloRing users (VTE incidence 8.3 per 10,000 WY) and women using COCs (VTE incidence 9.2 per 10,000 WY). For women using COCs that did not contain the progestins desogestrel (DSG) or gestodene (GSD), VTE incidence was 8.9 per 10,000 WY.
A retrospective cohort study using data from 4 health plans in the US (FDA-funded Study in Kaiser Permanente and Medicaid databases) showed the VTE incidence for new users of EnilloRing to be 11.4 events per 10,000 WY, for new users of a levonorgestrel (LNG)-containing COC 9.2 events per 10,000 WY, and for users of other COCs available during the course of the study* 8.2 events per 10,000 WY.
*Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel.
Table 1: Estimates (Hazard Ratios) of Venous Thromboembolism Risk in Users of EnilloRing Compared to Users of Combined Oral Contraceptives (COCs)Epidemiologic Study(Author, Year of Publication) Population StudiedComparator Product(s)Hazard Ratios (HR)(95% CI)TASC (Dinger, 2012) Initiators, including new users, switchers and restarters
All COCs available during the course of the study * COCs available excluding DSG-or GSD -containing OCs
HR†: 0.8 (0.5-1.5)
HR†: 0.8 (0.4-1.7)
FDA-funded Study in Kaiser Permanente and Medicaid databases (Sidney, 2011)
First use of a combined hormonal contraceptive (CHC) during the study period
COCs available during the course of the study‡
LNG/0.03 mg ethinyl estradiol
HR§: 1.1 (0.6-2.2)
HR§: 1.0 (0.5-2.0)
* Includes low-dose COCs containing the following progestins: chlormadinone acetate, cyproterone acetate, desogestrel, dienogest, drospirenone, ethynodiol diacetate, gestodene, levonorgestrel, norethindrone, norgestimate, or norgestrel
† Adjusted for age, BMI, duration of use, VTE history
‡Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel
§Adjusted for age, site, year of entry into studyAn increased risk of thromboembolic and thrombotic disease associated with the use of CHCs is well-established. Although the absolute VTE rates are increased for users of CHCs compared to non-users, the rates associated with pregnancy are even greater, especially during the post-partum period (see Figure 1).
The frequency of VTE in women using CHCs has been estimated to be 3 to 12 cases per 10,000 women-years.
The risk of VTE is highest during the first year of CHC use and after restarting a CHC following a break of at least four weeks. The risk of VTE due to CHCs gradually disappears after use is discontinued.Figure 1 shows the risk of developing a VTE for women who are not pregnant and do not use CHCs, for women who use CHCs, for pregnant women, and for women in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 women who are not pregnant and do not use CHCs are followed for one year, between 1 and 5 of these women will develop a VTE.
Figure 1: Likelihood of Developing a VTE
*CHC=combination hormonal contraception
**Pregnancy data based on actual duration of pregnancy in the reference studies. Based on a model assumption that pregnancy duration is nine months, the rate is 7 to 27 per 10,000 WY.Several epidemiology studies indicate that third generation oral contraceptives, including those containing desogestrel (etonogestrel, the progestin in EnilloRing, is the biologically active metabolite of desogestrel), may be associated with a higher risk of VTE than oral contraceptives containing other progestins. Some of these studies indicate an approximate two-fold increased risk. However, data from other studies have not shown this two-fold increase in risk.
Use of CHCs also increases the risk of arterial thromboses such as strokes and myocardial infarctions, especially in women with other risk factors for these events. CHCs have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes). In general, the risk is greatest among older (>35 years of age), hypertensive women who also smoke.
Use EnilloRing with caution in women with cardiovascular disease risk factors.

image description - Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see Warnings and Precautions ()]5.1 Thromboembolic Disorders and Other Vascular Problems
Stop EnilloRing use if an arterial thrombotic or venous thromboembolic event (VTE) occurs. Stop EnilloRing use if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
[See Adverse Reactions .]If feasible, stop EnilloRing at least four weeks before and through two weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism, and during and following prolonged immobilization.
Start EnilloRing no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
The use of CHCs increases the risk of VTE. Known risk factors for VTE include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of CHCs
[see Contraindications ].Two epidemiologic studies1, 2, 3that assessed the risk of VTE associated with the use of EnilloRing are described below.
In these studies, which were required or sponsored by regulatory agencies, EnilloRing users had a risk of VTE similar to Combined Oral Contraceptives (COCs) users (see Table 1 for adjusted hazard ratios). A large prospective, observational study, the Transatlantic Active Surveillance on Cardiovascular Safety of EnilloRing (TASC), investigated the risk of VTE for new users, and women who were switching to or restarting EnilloRing or COCs in a population that is representative of routine clinical users. The women were followed for 24 to 48 months. The results showed a similar risk of VTE among EnilloRing users (VTE incidence 8.3 per 10,000 WY) and women using COCs (VTE incidence 9.2 per 10,000 WY). For women using COCs that did not contain the progestins desogestrel (DSG) or gestodene (GSD), VTE incidence was 8.9 per 10,000 WY.
A retrospective cohort study using data from 4 health plans in the US (FDA-funded Study in Kaiser Permanente and Medicaid databases) showed the VTE incidence for new users of EnilloRing to be 11.4 events per 10,000 WY, for new users of a levonorgestrel (LNG)-containing COC 9.2 events per 10,000 WY, and for users of other COCs available during the course of the study* 8.2 events per 10,000 WY.
*Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel.
Table 1: Estimates (Hazard Ratios) of Venous Thromboembolism Risk in Users of EnilloRing Compared to Users of Combined Oral Contraceptives (COCs)Epidemiologic Study(Author, Year of Publication) Population StudiedComparator Product(s)Hazard Ratios (HR)(95% CI)TASC (Dinger, 2012) Initiators, including new users, switchers and restarters
All COCs available during the course of the study * COCs available excluding DSG-or GSD -containing OCs
HR†: 0.8 (0.5-1.5)
HR†: 0.8 (0.4-1.7)
FDA-funded Study in Kaiser Permanente and Medicaid databases (Sidney, 2011)
First use of a combined hormonal contraceptive (CHC) during the study period
COCs available during the course of the study‡
LNG/0.03 mg ethinyl estradiol
HR§: 1.1 (0.6-2.2)
HR§: 1.0 (0.5-2.0)
* Includes low-dose COCs containing the following progestins: chlormadinone acetate, cyproterone acetate, desogestrel, dienogest, drospirenone, ethynodiol diacetate, gestodene, levonorgestrel, norethindrone, norgestimate, or norgestrel
† Adjusted for age, BMI, duration of use, VTE history
‡Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel
§Adjusted for age, site, year of entry into studyAn increased risk of thromboembolic and thrombotic disease associated with the use of CHCs is well-established. Although the absolute VTE rates are increased for users of CHCs compared to non-users, the rates associated with pregnancy are even greater, especially during the post-partum period (see Figure 1).
The frequency of VTE in women using CHCs has been estimated to be 3 to 12 cases per 10,000 women-years.
The risk of VTE is highest during the first year of CHC use and after restarting a CHC following a break of at least four weeks. The risk of VTE due to CHCs gradually disappears after use is discontinued.Figure 1 shows the risk of developing a VTE for women who are not pregnant and do not use CHCs, for women who use CHCs, for pregnant women, and for women in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 women who are not pregnant and do not use CHCs are followed for one year, between 1 and 5 of these women will develop a VTE.
Figure 1: Likelihood of Developing a VTE
*CHC=combination hormonal contraception
**Pregnancy data based on actual duration of pregnancy in the reference studies. Based on a model assumption that pregnancy duration is nine months, the rate is 7 to 27 per 10,000 WY.Several epidemiology studies indicate that third generation oral contraceptives, including those containing desogestrel (etonogestrel, the progestin in EnilloRing, is the biologically active metabolite of desogestrel), may be associated with a higher risk of VTE than oral contraceptives containing other progestins. Some of these studies indicate an approximate two-fold increased risk. However, data from other studies have not shown this two-fold increase in risk.
Use of CHCs also increases the risk of arterial thromboses such as strokes and myocardial infarctions, especially in women with other risk factors for these events. CHCs have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes). In general, the risk is greatest among older (>35 years of age), hypertensive women who also smoke.
Use EnilloRing with caution in women with cardiovascular disease risk factors.

image description - Have inherited or acquired hypercoagulopathies [see Warnings and Precautions ()]5.1 Thromboembolic Disorders and Other Vascular Problems
Stop EnilloRing use if an arterial thrombotic or venous thromboembolic event (VTE) occurs. Stop EnilloRing use if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
[See Adverse Reactions .]If feasible, stop EnilloRing at least four weeks before and through two weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism, and during and following prolonged immobilization.
Start EnilloRing no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
The use of CHCs increases the risk of VTE. Known risk factors for VTE include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of CHCs
[see Contraindications ].Two epidemiologic studies1, 2, 3that assessed the risk of VTE associated with the use of EnilloRing are described below.
In these studies, which were required or sponsored by regulatory agencies, EnilloRing users had a risk of VTE similar to Combined Oral Contraceptives (COCs) users (see Table 1 for adjusted hazard ratios). A large prospective, observational study, the Transatlantic Active Surveillance on Cardiovascular Safety of EnilloRing (TASC), investigated the risk of VTE for new users, and women who were switching to or restarting EnilloRing or COCs in a population that is representative of routine clinical users. The women were followed for 24 to 48 months. The results showed a similar risk of VTE among EnilloRing users (VTE incidence 8.3 per 10,000 WY) and women using COCs (VTE incidence 9.2 per 10,000 WY). For women using COCs that did not contain the progestins desogestrel (DSG) or gestodene (GSD), VTE incidence was 8.9 per 10,000 WY.
A retrospective cohort study using data from 4 health plans in the US (FDA-funded Study in Kaiser Permanente and Medicaid databases) showed the VTE incidence for new users of EnilloRing to be 11.4 events per 10,000 WY, for new users of a levonorgestrel (LNG)-containing COC 9.2 events per 10,000 WY, and for users of other COCs available during the course of the study* 8.2 events per 10,000 WY.
*Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel.
Table 1: Estimates (Hazard Ratios) of Venous Thromboembolism Risk in Users of EnilloRing Compared to Users of Combined Oral Contraceptives (COCs)Epidemiologic Study(Author, Year of Publication) Population StudiedComparator Product(s)Hazard Ratios (HR)(95% CI)TASC (Dinger, 2012) Initiators, including new users, switchers and restarters
All COCs available during the course of the study * COCs available excluding DSG-or GSD -containing OCs
HR†: 0.8 (0.5-1.5)
HR†: 0.8 (0.4-1.7)
FDA-funded Study in Kaiser Permanente and Medicaid databases (Sidney, 2011)
First use of a combined hormonal contraceptive (CHC) during the study period
COCs available during the course of the study‡
LNG/0.03 mg ethinyl estradiol
HR§: 1.1 (0.6-2.2)
HR§: 1.0 (0.5-2.0)
* Includes low-dose COCs containing the following progestins: chlormadinone acetate, cyproterone acetate, desogestrel, dienogest, drospirenone, ethynodiol diacetate, gestodene, levonorgestrel, norethindrone, norgestimate, or norgestrel
† Adjusted for age, BMI, duration of use, VTE history
‡Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel
§Adjusted for age, site, year of entry into studyAn increased risk of thromboembolic and thrombotic disease associated with the use of CHCs is well-established. Although the absolute VTE rates are increased for users of CHCs compared to non-users, the rates associated with pregnancy are even greater, especially during the post-partum period (see Figure 1).
The frequency of VTE in women using CHCs has been estimated to be 3 to 12 cases per 10,000 women-years.
The risk of VTE is highest during the first year of CHC use and after restarting a CHC following a break of at least four weeks. The risk of VTE due to CHCs gradually disappears after use is discontinued.Figure 1 shows the risk of developing a VTE for women who are not pregnant and do not use CHCs, for women who use CHCs, for pregnant women, and for women in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 women who are not pregnant and do not use CHCs are followed for one year, between 1 and 5 of these women will develop a VTE.
Figure 1: Likelihood of Developing a VTE
*CHC=combination hormonal contraception
**Pregnancy data based on actual duration of pregnancy in the reference studies. Based on a model assumption that pregnancy duration is nine months, the rate is 7 to 27 per 10,000 WY.Several epidemiology studies indicate that third generation oral contraceptives, including those containing desogestrel (etonogestrel, the progestin in EnilloRing, is the biologically active metabolite of desogestrel), may be associated with a higher risk of VTE than oral contraceptives containing other progestins. Some of these studies indicate an approximate two-fold increased risk. However, data from other studies have not shown this two-fold increase in risk.
Use of CHCs also increases the risk of arterial thromboses such as strokes and myocardial infarctions, especially in women with other risk factors for these events. CHCs have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes). In general, the risk is greatest among older (>35 years of age), hypertensive women who also smoke.
Use EnilloRing with caution in women with cardiovascular disease risk factors.

image description - Have uncontrolled hypertension [see Warnings and Precautions ()]5.5 High Blood Pressure
EnilloRing is contraindicated in women with uncontrolled hypertension or hypertension with vascular disease
[see Contraindications ]. For women with well-controlled hypertension, monitor blood pressure and stop EnilloRing use if blood pressure rises significantly.An increase in blood pressure has been reported in women using CHCs and this increase is more likely in older women and with extended duration of use. The incidence of hypertension increases with increasing concentrations of progestin.
- Have diabetes mellitus with vascular disease [see Warnings and Precautions ()]5.9 Carbohydrate and Lipid Metabolic Effects
Carefully monitor prediabetic and diabetic women who are using EnilloRing. CHCs may decrease glucose tolerance.
Consider alternative contraception for women with uncontrolled dyslipidemia. Some women will have adverse lipid changes while on CHCs.
Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using CHCs. - Have headaches with focal neurological symptoms or migraine headaches with aura [see Warnings and Precautions ()]5.10 Headache
If a woman using EnilloRing develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue EnilloRing if indicated.
Consider discontinuation of EnilloRing in the case of an increased frequency or severity of migraine during CHC use (which may be prodromal of a cerebrovascular event)[see Contraindications ].- Women over age 35 with any migraine headaches [see Warnings and Precautions ()]5.10 Headache
If a woman using EnilloRing develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue EnilloRing if indicated.
Consider discontinuation of EnilloRing in the case of an increased frequency or severity of migraine during CHC use (which may be prodromal of a cerebrovascular event)[see Contraindications ].
- Women over age 35 with any migraine headaches
- Smoke, if over age 35
- Liver tumors, benign or malignant, or liver disease [see Warnings and Precautions ()and Use in Specific Populations (5.3 Liver DiseaseImpaired Liver Function
Do not use EnilloRing in women with liver disease such as acute viral hepatitis or severe (decompensated) cirrhosis of the liver
[see Contraindications ].Acute or chronic disturbances of liver function may necessitate the discontinuation of CHC use until markers of liver function return to normal and CHC causation has been excluded[see Use in Specific Populations ].Discontinue EnilloRing use if jaundice develops.Liver TumorsEnilloRing is contraindicated in women with benign and malignant liver tumors
[see Contraindications ].Hepatic adenomas are associated with CHC use. An estimate of the attributable risk is 3.3 cases per 100,000 CHC users. Rupture of hepatic adenomas may cause death through intra-abdominal hemorrhage.Studies have shown an increased risk of developing hepatocellular carcinoma in long term (>8 years) CHC users. However, the attributable risk of liver cancers in CHC users is less than one case per million users.
)]8.6 Hepatic ImpairmentThe effect of hepatic impairment on the pharmacokinetics of EnilloRing has not been studied. Steroid hormones may be poorly metabolized in patients with impaired liver function. Acute or chronic disturbances of liver function may necessitate the discontinuation of CHC use until markers of liver function return to normal. [
See Contraindications and Warnings and Precautions.] - Undiagnosed abnormal uterine bleeding[see Warnings and Precautions ()]5.11 Bleeding Irregularities and AmenorrheaUnscheduled Bleeding and Spotting
Unscheduled bleeding (breakthrough or intracyclic) bleeding and spotting sometimes occur in women using CHCs, especially during the first three months of use. If bleeding persists or occurs after previously regular cycles, check for causes such as pregnancy or malignancy. If pathology and pregnancy are excluded, bleeding irregularities may resolve over time or with a change to a different CHC.
Bleeding patterns were evaluated in three large clinical studies. In the North American study (US and Canada, N=1,177), the percentages of subjects with breakthrough bleeding/spotting ranged from 7.2% to 11.7% during cycles 1-13. In the two non-US studies, the percentages of subjects with breakthrough bleeding/spotting ranged from 2.6% to 6.4% (Europe, N=1,145) and from 2.0% to 8.7% (Europe, Brazil, Chile, N=512).Amenorrhea and Oligomenorrhea
If scheduled (withdrawal) bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule, consider the possibility of pregnancy at the time of the first missed period and take appropriate diagnostic measures.
Occasional missed periods may occur with the appropriate use of EnilloRing. In the clinical studies, the percent of women who did not have withdrawal bleeding in a given cycle ranged from 0.3% to 3.8%.
If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy.
Some women may experience amenorrhea or oligomenorrhea after discontinuing CHC use, especially when such a condition was pre-existent. - Pregnancy, because there is no reason to use CHCs during pregnancy [see Use in Specific Populations ()]8.1 PregnancyRisk Summary
EnilloRing is contraindicated during pregnancy because there is no need for pregnancy prevention in a woman who is already pregnant. Epidemiologic studies and meta-analyses have not shown an increased risk of genital or non-genital birth defects (including cardiac anomalies and limb-reduction defects) following maternal exposure to low dose CHCs prior to conception or during early pregnancy. No adverse developmental outcomes were observed in pregnant rats and rabbits with the administration of etonogestrel during organogenesis at doses approximately 300 times the anticipated daily vaginal human dose (~0.002 mg/kg/day).No adverse developmental outcomes were observed in pregnant rats and rabbits with the co-administration of the combination desogestrel/ethinyl estradiol during organogenesis at desogestrel/ethinyl estradiol doses at least 2/5 times, respectively, the anticipated daily vaginal human dose (~0.002 desogestrel/0.00025 ethinyl estradiol mg/kg/day).
Discontinue EnilloRing use if pregnancy is confirmed.
DataAnimal Data
In rats and rabbits at dosages up to 300 times the anticipated dose, etonogestrel is neither embryotoxic nor teratogenic. Co-administration of a maternally toxic dose of desogestrel/ethinyl estradiol to pregnant rats was associated with embryolethality and wavy ribs at a desogestrel/ethinyl estradiol dose that was 40/130 times, respectively, the anticipated vaginal human dose (0.002 desogestrel/0.00025 ethinyl estradiol mg/kg/day). No adverse embryofetal effects were observed when the combination was administered to pregnant rats at a desogestrel/ethinyl estradiol dose that was 4/13 times, respectively, the anticipated vaginal human dose. When desogestrel/ethinyl estradiol was given to pregnant rabbits, preimplantation loss was observed at a desogestrel/ethinyl estradiol dose that was 3/10 times, respectively, the anticipated vaginal human dose. No adverse embryofetal effects were observed when the combination was administered to pregnant rabbits at a desogestrel/ethinyl estradiol dose that was 2/5 times the anticipated vaginal human dose. Current diagnosis of, or history of, breast cancer, which may be hormone-sensitive
[see Warnings and Precautions ()]5.14 Malignant NeoplasmsBreast Cancer
EnilloRing is contraindicated in females who currently have or have had breast cancer because breast cancer may be hormonally sensitive[see Contraindications ].
Epidemiology studies have not found a consistent association between use of combined oral contraceptives (COCs) and breast cancer risk. Studies do not show an association between ever (current or past) use of COCs and risk of breast cancer. However, some studies report a small increase in the risk of breast cancer among current or recent users (<6 months since last use) and current users with longer duration of COC use[see Postmarketing Experience ].Cervical Cancer
Some studies suggest that CHCs are associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there is controversy about the extent to which these findings may be due to differences in sexual behavior and other factors.- Hypersensitivity reactions, including anaphylaxis and angioedema, to any of the components of EnilloRing [see Warnings and Precautions () and Adverse Reactions (5.6 Hypersensitivity Reactions
Hypersensitivity reactions of anaphylaxis and angioedema have been reported during use of EnilloRing. If anaphylaxis and/or angioedema is suspected, EnilloRing should be discontinued and appropriate treatment administered.
[see Contraindications .])]6 ADVERSE REACTIONSThe following serious adverse reactions with the use of CHCs are discussed elsewhere in the labeling.
- Serious cardiovascular events and stroke [see Boxed Warningand Warnings and Precautions]
- Vascular events [see Warnings and Precautions]
- Liver disease [see Warnings and Precautions]
Adverse reactions commonly reported by CHC users are:
- Irregular uterine bleeding
- Nausea
- Breast tenderness
- Headache
The most common adverse reactions (≥2%) in clinical trials were: vaginitis, headache (including migraine), mood changes (e.g., depression, mood swings, mood altered, depressed mood, affect lability), device-related events (e.g., expulsion/discomfort/foreign body sensation), nausea/vomiting, vaginal discharge, increased weight, vaginal discomfort, breast pain/discomfort/tenderness, dysmenorrhea, abdominal pain, acne, and decreased libido.
To report SUSPECTED ADVERSE REACTIONS, contact Xiromed, LLC at 844-XIROMED (844-947-6633)or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Trials with a duration of 6 to 13 28-day cycles provided safety data. In total, 2,501 women, aged 18 to 41 contributed 24,520 cycles of exposure.
Common Adverse Reactions (≥ 2%):vaginitis (13.8%), headache (including migraine) (11.2%), mood changes (e.g., depression, mood swings, mood altered, depressed mood, affect lability) (6.4%), device-related events (e.g., expulsion/discomfort/foreign body sensation) (6.3%), nausea/vomiting (5.9%), vaginal discharge (5.7%), increased weight (4.9%), vaginal discomfort (4.0%), breast pain/discomfort/tenderness (3.8%), dysmenorrhea (3.5%), abdominal pain (3.2%), acne (2.4%), and decreased libido (2.0%).Adverse Reactions (≥ 1%) Leading to Study Discontinuation:13.0% of the women discontinued from the clinical trials due to an adverse reaction; the most common adverse reactions leading to discontinuation were device-related events (2.7%), mood changes (1.7%), headache (including migraine) (1.5%) and vaginal symptoms (1.2%).Serious Adverse Reactions:deep vein thrombosis [see Warnings and Precautions], anxiety, cholelithiasis, and vomiting.6.2 Postmarketing ExperienceFive studies that compared breast cancer risk between ever-users (current or past use) of COCs and never-users of COCs reported no association between ever use of COCs and breast cancer risk, with effect estimates ranging from 0.90 - 1.12 (Figure 2).
Three studies compared breast cancer risk between current or recent COC users (<6 months since last use) and never users of COCs (Figure 2). One of these studies reported no association between breast cancer risk and COC use. The other two studies found an increased relative risk of 1.19 - 1.33 with current or recent use. Both of these studies found an increased risk of breast cancer with current use of longer duration, with relative risks ranging from 1.03 with less than one year of COC use to approximately 1.4 with more than 8-10 years of COC use.Figure 2: Relevant Studies of Risk of Breast Cancer with Combined Oral Contraceptives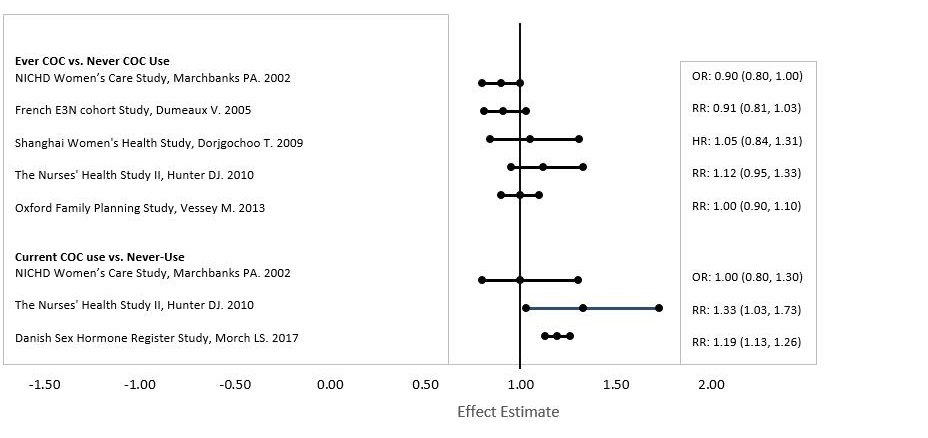
RR = relative risk; OR = odds ratio; HR = hazard ratio. “ever COC” are females with current or past COC use; “never COC use” are
females that never used COCs.The following adverse reactions have been identified during post-approval use of EnilloRing. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: hypersensitivity reactions, including anaphylaxis and angioedemaNervous system disorders: stroke/cerebrovascular accidentVascular disorders: arterial events (including arterial thromboembolism and myocardial infarction), aggravation of varicose veinsSkin and subcutaneous tissue disorders: exacerbations of hereditary and acquired angioedema, urticaria, chloasmaReproductive system and breast disorders: penile disorders, including local reactions on penis (in male partners of women usingEnilloRing ), galactorrheaGeneral Disorders and Administration Site Conditions: device breakage (including with concomitant use of intravaginal antimycotic, antibiotic, and lubricant products)Injury, poisoning and procedural complications: vaginal injury (including associated pain, discomfort, and bleeding) associated with ring breakage
enilloring-chart - Serious cardiovascular events and stroke [
- Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to potential for ALT elevations[see Warnings and Precautions ()].5.4 Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
CHCs, such as EnilloRing, are contraindicated for use with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir (with or without dasabuvir)
[see Contraindications ]. Discontinue EnilloRing prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir (with or without dasabuvir). EnilloRing can be restarted approximately 2 weeks following completion of treatment with this hepatitis C combination drug regimen.During clinical trials with some HCV combination drug regimens, ALT elevations were observed in women using ethinyl estradiol containing medications
[see Drug Interactions ]. For example, the Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications, such as CHCs.