Enoxaparin Sodium Prescribing Information
- Use of indwelling epidural catheters
- Concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, and other anticoagulants
- A history of traumatic or repeated epidural or spinal punctures
- A history of spinal deformity or spinal surgery
- Optimal timing between the administration of Enoxaparin Sodium Injection and neuraxial procedures is not known.
Cases of epidural or spinal hemorrhage and subsequent hematomas have been reported with the use of Enoxaparin Sodium Injection and epidural or spinal anesthesia/analgesia or spinal puncture procedures, resulting in long-term or permanent paralysis. The risk of these events is higher with the use of postoperative indwelling epidural catheters, with the concomitant use of additional drugs affecting hemostasis such as NSAIDs, with traumatic or repeated epidural or spinal puncture, or in patients with a history of spinal surgery or spinal deformity [
To reduce the potential risk of bleeding associated with the concurrent use of Enoxaparin Sodium Injection and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of Enoxaparin Sodium Injection [
Placement or removal of an epidural catheter or lumbar puncture is best performed when the anticoagulant effect of Enoxaparin Sodium Injection is low; however, the exact timing to reach a sufficiently low anticoagulant effect in each patient is not known.
Placement or removal of a catheter should be delayed for at least 12 hours after administration of lower doses (30 mg once or twice-daily or 40 mg once daily) of Enoxaparin Sodium Injection, and at least 24 hours after the administration of higher doses (0.75 mg/kg twice daily, 1 mg/kg twice daily, or 1.5 mg/kg once daily) of Enoxaparin Sodium Injection.
Anti-Xa levels are still detectable at these time points, and these delays are not a guarantee that neuraxial hematoma will be avoided. Patients receiving the 0.75 mg/kg twice-daily dose or the 1 mg/kg twice daily dose should not receive the second Enoxaparin Sodium Injection dose in the twice-daily regimen to allow a longer delay before catheter placement or removal.
Likewise, although a specific recommendation for timing of a subsequent Enoxaparin Sodium Injection dose after catheter removal cannot be made, consider delaying this next dose for at least four hours, based on a benefit-risk assessment considering both the risk for thrombosis and the risk for bleeding in the context of the procedure and patient risk factors. For patients with creatinine clearance <30mL/minute, additional considerations are necessary because elimination of Enoxaparin Sodium Injection is more prolonged; consider doubling the timing of removal of a catheter, at least 24 hours for the lower prescribed dose of Enoxaparin Sodium Injection (30 mg once daily) and at least 48 hours for the higher dose (1 mg/kg/day) [
Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, frequent monitoring must be exercised to detect any signs and symptoms of neurological impairment such as midline back pain, sensory and
motor deficits (numbness or weakness in lower limbs), and bowel and/or bladder dysfunction.
Instruct patients to report immediately if they experience any of the above signs or symptoms.
If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
Use Enoxaparin Sodium Injection with extreme caution in conditions with increased risk of hemorrhage, such as bacterial endocarditis, congenital or acquired bleeding disorders, active ulcerative and angiodysplastic gastrointestinal disease, hemorrhagic stroke, or shortly after brain, spinal, or ophthalmological surgery, or in patients treated concomitantly with platelet
inhibitors.
Major hemorrhages including retroperitoneal and intracranial bleeding have been reported.
Some of these cases have been fatal.
Bleeding can occur at any site during therapy with Enoxaparin Sodium Injection. An unexplained fall in hematocrit or blood pressure should lead to a search for a bleeding site.
Whenever possible, agents which may enhance the risk of hemorrhage should be discontinued prior to initiation of Enoxaparin Sodium Injection therapy. These agents include medications such as: anticoagulants, platelet inhibitors including acetylsalicylic acid, salicylates, NSAIDs (including ketorolac tromethamine), dipyridamole, or sulfinpyrazone. If co-administration is essential, conduct close clinical and laboratory monitoring [
Discontinue agents which may enhance hemorrhage risk prior to initiation of Enoxaparin Sodium Injection or conduct close clinical and laboratory monitoring.
Enoxaparin Sodium Injection is a low molecular weight heparin (LMWH) indicated for:
- Prophylaxis of deep vein thrombosis (DVT) in abdominal surgery, hip replacement surgery, knee replacement surgery, or medical patients with severely restricted mobility during acute illness ()
1.1 Prophylaxis of Deep Vein ThrombosisEnoxaparin Sodium Injection is indicated for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE):
- in patients undergoing abdominal surgery who are at risk for thromboembolic complications [seeClinical Studies]
- in patients undergoing hip replacement surgery, during and following hospitalization
- in patients undergoing knee replacement surgery
- in medical patients who are at risk for thromboembolic complications due to severely restricted mobility during acute illness
- in patients undergoing abdominal surgery who are at risk for thromboembolic complications [see
- Inpatient treatment of acute DVT with or without pulmonary embolism ()
1.2 Treatment of Acute Deep Vein ThrombosisEnoxaparin Sodium Injection is indicated for:
- theinpatient treatmentof acute deep vein thrombosis with or without pulmonary embolism, when administered in conjunction with warfarin sodium
- theoutpatient treatmentof acute deep vein thrombosis without pulmonary embolism when administered in conjunction with warfarin sodium
- the
- Outpatient treatment of acute DVT without pulmonary embolism ()
1.2 Treatment of Acute Deep Vein ThrombosisEnoxaparin Sodium Injection is indicated for:
- theinpatient treatmentof acute deep vein thrombosis with or without pulmonary embolism, when administered in conjunction with warfarin sodium
- theoutpatient treatmentof acute deep vein thrombosis without pulmonary embolism when administered in conjunction with warfarin sodium
- the
- Prophylaxis of ischemic complications of unstable angina and non-Q-wave myocardial infarction (MI) ()
1.3 Prophylaxis of Ischemic Complications of Unstable Angina and Non-Q-Wave Myocardial InfarctionEnoxaparin Sodium Injection is indicated for the prophylaxis of ischemic complications of unstable angina and non-Q-wave myocardial infarction, when concurrently administered with aspirin.
- Treatment of acute ST-segment elevation myocardial infarction (STEMI) managed medically or with subsequent percutaneous coronary intervention (PCI) ()
1.4 Treatment of Acute ST-Segment Elevation Myocardial InfarctionEnoxaparin Sodium Injection, when administered concurrently with aspirin, has been shown to reduce the rate of the combined endpoint of recurrent myocardial infarction or death in patients with acute ST-segment elevation myocardial infarction (STEMI) receiving thrombolysis and being managed medically or with percutaneous coronary intervention (PCI).
See full prescribing information for dosing and administration information. (
See full prescribing information for dosing and administration information.
Evaluate all patients for a bleeding disorder before starting Enoxaparin Sodium Injection treatment, unless treatment is urgently needed.
The recommended dose of Enoxaparin Sodium Injection is
The recommended dose of Enoxaparin Sodium Injection is
A dose of Enoxaparin Sodium Injection of
The recommended dose of Enoxaparin Sodium Injection is
The recommended dose of Enoxaparin Sodium Injection is
The recommended dose of Enoxaparin Sodium Injection is 1 mg/kg every 12 hours administered subcutaneously
In both outpatient and inpatient (hospital) treatments, initiate warfarin sodium therapy when appropriate (usually within 72 hours of Enoxaparin Sodium Injection). Continue Enoxaparin Sodium Injection for a minimum of 5 days and until a therapeutic oral anticoagulant effect has been achieved (International Normalization Ratio 2 to 3). The average duration of administration is 7 days [
The recommended dose of Enoxaparin Sodium Injection is
The recommended dose of Enoxaparin Sodium Injection is a
When administered in conjunction with a thrombolytic (fibrin specific or non–fibrin specific), administer Enoxaparin Sodium Injection between 15 minutes before and 30 minutes after the start of fibrinolytic therapy. The usual duration of Enoxaparin Sodium Injection therapy is 8 days or until hospital discharge.
For patients managed with percutaneous coronary intervention (PCI), if the last Enoxaparin Sodium Injection subcutaneous administration was given less than 8 hours before balloon inflation, no additional dosing is needed. If the last Enoxaparin Sodium Injection subcutaneous administration was given more than 8 hours before balloon inflation, administer an intravenous bolus of 0.3 mg/kg of Enoxaparin Sodium Injection [
The recommended prophylaxis and treatment dosage regimens for patients with severe renal impairment (creatinine clearance <30 mL/min) are described in Table 1 [
Indication | Dosage Regimen |
Prophylaxis in abdominal surgery | 30 mg administered subcutaneously once daily |
Prophylaxis in hip or knee replacement surgery | 30 mg administered subcutaneously once daily |
Prophylaxis in medical patients during acute illness | 30 mg administered subcutaneously once daily |
Inpatient treatment of acute deep vein thrombosis with or without pulmonary embolism, when administered in conjunction with warfarin sodium | 1 mg/kg administered subcutaneously once daily |
Outpatient treatment of acute deep vein thrombosis without pulmonary embolism, when administered in conjunction with warfarin sodium | 1 mg/kg administered subcutaneously once daily |
Prophylaxis of ischemic complications of unstable angina and non–Q-wave myocardial infarction, when concurrently administered with aspirin | 1 mg/kg administered subcutaneously once daily |
Treatment of acute ST-segment elevation myocardial infarction in patients <75 years of age, when administered in conjunction with aspirin | 30 mg single intravenous bolus plus a 1 mg/kg subcutaneous dose followed by 1 mg/kg administered subcutaneously once daily |
Treatment of acute ST-segment elevation myocardial infarction in geriatric patients ≥75 years of age, when administered in conjunction with aspirin | 1 mg/kg administered subcutaneously once daily |
Although no dose adjustment is recommended in patients with creatinine clearance 30 to 50 mL/min and creatinine clearance 50 to 80 mL/min, observe these patients frequently for signs and symptoms of bleeding.
For treatment of acute ST-segment elevation myocardial infarction in geriatric patients ≥75 years of age,
No dose adjustment is necessary for other indications in geriatric patients unless kidney function is impaired [
Do not administer Enoxaparin Sodium Injection by intramuscular injection.
Administer Enoxaparin Sodium Injection by intravenous or subcutaneous injection only.
Enoxaparin Sodium Injection is a clear, colorless to pale yellow sterile solution, and as
with other parenteral drug products, should be inspected visually for particulate matter and
discoloration prior to administration.
Use a tuberculin syringe or equivalent when using Enoxaparin Sodium Injection multiple-dose
vials to assure withdrawal of the appropriate volume of drug.
Patients may self-inject by the subcutaneous route of administration only after their physicians
determine that it is appropriate and with medical follow-up, as necessary. Provide proper training in subcutaneous injection technique before allowing self-injection (with or without the
assistance of an injection device).
- Position patients in a supine position for Enoxaparin Sodium Injection administration by deep subcutaneous injection.
- Do not expel the air bubble from the prefilled syringes before the injection, to avoid the loss of drug.
- Do not inject into skin that has bruises or scars. Do not inject through clothes.
- Alternate injection sites between the left and right anterolateral and left and right posterolateral abdominal wall.
- Introduce the whole length of the needle into a skin fold held between the thumb and forefinger; hold the skin fold throughout the injection. To minimize bruising, do not rub the injection site after completion of the injection.
Enoxaparin Sodium Injection prefilled syringes and graduated prefilled syringes are for single, one-time use only and are available with a system that shields the needle after injection.
Remove the prefilled syringe from the blister packaging by peeling the lid completely off at the arrow as directed on the blister. Then gently lift the syringe straight up by grasping either the middle of the syringe or the needle guard. Do not remove by pulling on the plunger as this
may damage the syringe.
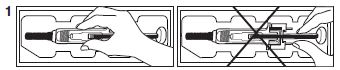
- Peel lid completely off
- Do not remove syringe by pulling on plunger
- Gently lift syringe straight up
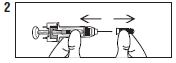 | Remove needle cover by pulling straight off |
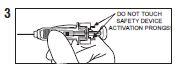 | Administer by subcutaneous (SC) injection as instructed by your pharmacist or health- care provider. |
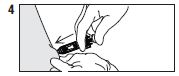 | Push the plunger with slow constant pressure plunger all the way down as far as it will go to |
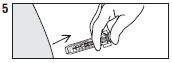 | Remove needle from injection site, then let |
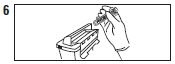 | Dispose of syringe / needle guard assembly |
NOTE:
- The safety system should only activate once the syringe has been emptied.
- Activation of the safety system must be done only after removing the needle from the patient’s skin.
- Do not replace the needle shield after injection.
- The safety system should not be sterilized.
Activation of the safety system may cause minimal splatter of fluid. For optimal safety activate the system while orienting it downwards away from yourself and others.
Use the multiple-dose vial for intravenous injections. Administer Enoxaparin Sodium Injection through an intravenous line. Do not mix or coadminister Enoxaparin Sodium Injection with other medications. Flush the intravenous access device with a sufficient volume of saline or dextrose solution prior to and following the intravenous bolus administration of Enoxaparin Sodium Injection, to prevent mixing of drugs. Enoxaparin Sodium Injection is compatible with normal saline solution (0.9%) or 5% dextrose in water.






During therapy monitor complete blood counts including platelets and stool occult blood.
Assess for signs and symptoms of bleeding.
In patients with renal impairment anti-Factor Xa levels may be used to monitor the anticoagulant effects of Enoxaparin Sodium Injection.
If during Enoxaparin Sodium Injection therapy abnormal coagulation parameters or bleeding should occur, anti-Factor Xa levels may be used to monitor the anticoagulant effects of Enoxaparin Sodium Injection [
Prothrombin Time (PT) and Activated Partial Thromboplastin Time (aPTT) are not adequate for monitoring the anticoagulant effects of Enoxaparin Sodium Injection.
Enoxaparin Sodium Injection USP is a clear, colorless to pale yellow solution available in two concentrations:
-Single-Dose Prefilled Syringes | 30 mg / 0.3 mL, 40 mg / 0.4 mL |
-Single-Dose Graduated Prefilled Syringes | 60 mg / 0.6 mL, 80 mg / 0.8 mL, 100 mg / 1 mL |
-Multiple-Dose Vials | 300 mg / 3 mL |
-Single-Dose Graduated Prefilled Syringes | 120 mg / 0.8 mL, 150 mg / 1 mL |
• Severe Renal Impairment: Adjust dose for patients with creatinine clearance <30 mL/min. (
In patients with renal impairment, there is an increase in exposure of enoxaparin sodium. All such patients should be observed carefully for signs and symptoms of bleeding. Because exposure of enoxaparin sodium is significantly increased in patients with severe renal impairment (creatinine clearance <30 mL/min), a dosage adjustment is recommended for therapeutic and prophylactic dosage ranges. No dosage adjustment is recommended in patients with creatinine clearance 30 to <50 mL/min and creatinine clearance 50 to 80 mL/min [
• Geriatric Patients: Monitor for increased risk of bleeding. (
Over 2800 patients, 65 years and older, have received Enoxaparin Sodium Injection in clinical trials. The efficacy of Enoxaparin Sodium Injection in the geriatric (≥65 years) was similar to that seen in younger patients (<65 years). The incidence of bleeding complications was similar between geriatric and younger patients when 30 mg every 12 hours or 40 mg once a day doses of Enoxaparin Sodium Injection were employed. The incidence of bleeding complications was higher in geriatric patients as compared to younger patients when Enoxaparin Sodium Injection was administered at doses of 1.5 mg/kg once a day or 1 mg/kg every 12 hours. The risk of Enoxaparin Sodium Injection-associated bleeding increased with age. Serious adverse events increased with age for patients receiving Enoxaparin Sodium Injection. Other clinical experience (including postmarketing surveillance and literature reports) has not revealed additional differences in the safety of Enoxaparin Sodium Injection between geriatric and younger patients. Careful attention to dosing intervals and concomitant medications (especially antiplatelet medications) is advised. Enoxaparin Sodium Injection should be used with care in geriatric patients who may show delayed elimination of enoxaparin. Monitoring of geriatric patients with low body weight (<45 kg) and those predisposed to decreased renal function should be considered [
In the clinical study for treatment of acute ST-segment elevation myocardial infarction, there was no evidence of difference in efficacy between patients ≥75 years of age (n = 1241) and patients less than 75 years of age (n = 9015). Patients ≥75 years of age did not receive a 30 mg intravenous bolus prior to the normal dosage regimen and had their subcutaneous dose adjusted to 0.75 mg/kg every 12 hours [
• Low-Weight Patients: Observe for signs of bleeding. (
An increase in exposure of enoxaparin sodium with prophylactic dosages (non-weight adjusted) has been observed in low-weight women (<45 kg) and low-weight men (<57 kg). Observe low-weight patients frequently for signs and symptoms of bleeding [
Enoxaparin Sodium Injection is contraindicated in patients with:
- Active major bleeding
- History of immune-mediated heparin-induced thrombocytopenia (HIT) within the past 100 days or in the presence of circulating antibodies [see Warnings and Precautions (])
5.4 Risk of Heparin-Induced Thrombocytopenia with or without ThrombosisEnoxaparin Sodium Injection may cause Heparin-Induced Thrombocytopenia (HIT) or Heparin-Induced Thrombocytopenia with Thrombosis (HITTS). HITTS may lead to organ infarction, limb ischemia, or death. Monitor thrombocytopenia of any degree closely.
Use of Enoxaparin Sodium Injection in patients with a history of immune-mediated HIT within the past 100 days or in the presence of circulating antibodies is contraindicated [see Contraindications].
Circulating antibodies may persist for several years.
Only use Enoxaparin Sodium Injection in patients with a history of HIT, if more than 100 days have elapsed since the prior HIT episode and no circulating antibodies are present. Because HIT may still occur in these circumstances, the decision to use Enoxaparin Sodium Injection
in such a case must be made only after a careful benefit-risk assessment and after nonheparin alternative treatments are considered. - Known hypersensitivity to enoxaparin sodium (e.g., pruritus, urticaria, anaphylactic/ anaphylactoid reactions) [see Adverse Reactions (])
6.2 Postmarketing ExperienceThe following adverse reactions have been identified during postapproval use of Enoxaparin Sodium Injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
There have been reports of epidural or spinal hematoma formation with concurrent use of Enoxaparin Sodium Injection and spinal/epidural anesthesia or spinal puncture. The majority of patients had a postoperative indwelling epidural catheter placed for analgesia or received additional drugs affecting hemostasis such as NSAIDs. Many of the epidural or spinal hematomas caused neurologic injury, including long-term or permanent paralysis.
Local reactions at the injection site (e.g., nodules, inflammation, oozing), systemic allergic reactions (e.g., pruritus, urticaria, anaphylactic/anaphylactoid reactions including shock), vesiculobullous rash, rare cases of hypersensitivity cutaneous vasculitis, purpura, skin necrosis (occurring at either the injection site or distant from the injection site), thrombocytosis, and thrombocytopenia with thrombosis [see Warnings and Precautions] have been reported.
Cases of hyperkalemia have been reported. Most of these reports occurred in patients who also had conditions that tend toward the development of hyperkalemia (e.g., renal dysfunction, concomitant potassium-sparing drugs, administration of potassium, hematoma in body tissues). Very rare cases of hyperlipidemia have also been reported, with one case of hyperlipidemia, with marked hypertriglyceridemia, reported in a diabetic pregnant woman; causality has not been determined.
Cases of headache, hemorrhagic anemia, eosinophilia, alopecia, hepatocellular and cholestatic liver injury have been reported.
Osteoporosis has also been reported following long-term therapy. - Known hypersensitivity to heparin or pork products
- Known hypersensitivity to benzyl alcohol (which is in only the multiple-dose formulation of Enoxaparin Sodium Injection [see Warnings and Precautions (5.8)]