Epoprostenol
Epoprostenol Prescribing Information
Epoprostenol for injection is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise capacity. Studies establishing effectiveness included predominantly patients with NYHA Functional Class III-IV symptoms and etiologies of idiopathic or heritable PAH or PAH associated with connective tissue diseases.
Important Note:
Reconstitute Epoprostenol for Injection only as directed with Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP. Do not dilute reconstituted solutions of epoprostenol for injection or administer it with other parenteral solutions or medicationsEpoprostenol for Injection is
Use after reconstitution and immediate dilution to final concentration
Use at room temperature (77°F/25°C)
Epoprostenol for Injection
solution reconstituted with 5 mL of Sterile Water for Injection, USP or Sodium Chloride 0.9% Injection,and immediately diluted to the final concentration in the drug delivery reservoir can be administered at room temperature per the conditions of use as outlined in Table 1.Final concentration range | Immediate administration | If stored for up to 8 days at 36° to 46°F (2° to 8°C) |
0.5 mg vial | ||
≥3,000 ng/mL and <15,000 ng/mL | 48 hours | 24 hours |
1.5 mg vial | ||
≥15,000 ng/mL and | 48 hours | 48 hours |
≥60,000 ng/mL | 72 hours | 48 hours |
Temperatures greater than 77°F and up to 86°F (>25
Temperatures up to 104°F (40
Do not expose this solution to direct sunlight.
A concentration for the solution of epoprostenol for injection
should be selected that is compatible with the infusion pump being used with respect to minimum and maximum flow rates, reservoir capacity, and the infusion pump criteria listed above. Epoprostenol for injection, when administered chronically, should be prepared in a drug delivery reservoir appropriate for the infusion pump. Outlined in Table 2 are directions for preparing different concentrations of epoprostenol for injection.Each vial is for single-dose; discard any unused solution.
To make 100 mL of solution with Final Concentration (ng/mL) of: | Directions: |
Using the 0.5 mg vial | |
3,000 ng/mL | Dissolve contents of one 0.5 mg vial with 5 mL of Sterile Water for Injection, USP or Sodium Chloride 0.9% Injection, USP. Withdraw 3 mL of the vial contents and add to a sufficient volume of the identical diluent to make a total of 100 mL. |
5,000 ng/mL | Dissolve contents of one 0.5 mg vial with 5 mL of Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP.Withdraw entire vial contents and add to a sufficient volume of the identical diluent to make a total of 100 mL. |
10,000 ng/mL | Dissolve contents of two 0.5 mg vials each with 5 mL of Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP.Withdraw entire vial contents and add to a sufficient volume of the identical diluent to make a total of 100 mL. |
Using the 1.5 mg vial | |
15,000 ng/mLHigher concentrations may be prepared for patients who receive epoprostenol for injection long-term. | Dissolve contents of one 1.5 mg vial with 5 mL of Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP.Withdraw entire vial contents and add to a sufficient volume of the identical diluent to make a total of 100 mL. |
30,000 ng/mL | Dissolve contents of two 1.5 mg vials each with 5 mL of Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP.Withdraw entire vial contents and add to a sufficient volume of the identical diluent to make a total of 100 mL. |
Infusion rates may be calculated using the following formula:
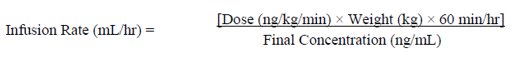
Tables 3 to 7 provide infusion delivery rates for doses up to 16 ng/kg/min based upon patient weight, drug delivery rate, and concentration of the solution of epoprostenol for injection to be used. These tables may be used to select the most appropriate concentration of epoprostenol for injection that will result in an infusion rate between the minimum and maximum flow rates of the infusion pump and that will allow the desired duration of infusion from a given reservoir volume. For infusion/dose rates lower than those listed in Tables 3 to 7, it is recommended that the pump rate be set by a healthcare professional such that steady state is achieved in the patient, keeping in mind the half-life of epoprostenol is no more than six minutes. Higher infusion rates, and therefore, more concentrated solutions may be necessary with long-term administration of epoprostenol for injection.
Patient weight (kg) | Dose or Drug Delivery Rate (ng/kg/min) | |||
2 | 3 | 4 | 5 | |
Infusion Delivery Rate (mL/hr) | ||||
20 | --- | 1.2 | 1.6 | 2 |
30 | 1.2 | 1.8 | 2.4 | 3 |
40 | 1.6 | 2.4 | 3.2 | 4 |
50 | 2 | 3 | 4 | --- |
60 | 2.4 | 3.6 | --- | --- |
70 | 2.8 | --- | --- | --- |
80 | 3.2 | --- | --- | --- |
90 | 3.6 | --- | --- | --- |
100 | 4 | --- | --- | --- |
Patient weight (kg) | Dose or Drug Delivery Rate (ng/kg/min) | ||||||
2 | 4 | 6 | 8 | 10 | 12 | 14 | |
Infusion Delivery Rate (mL/hr) | |||||||
20 | --- | 1 | 1.4 | 1.9 | 2.4 | 2.9 | 3.4 |
30 | --- | 1.4 | 2.2 | 2.9 | 3.6 | --- | --- |
40 | 1 | 1.9 | 2.9 | 3.8 | --- | --- | --- |
50 | 1.2 | 2.4 | 3.6 | --- | --- | --- | --- |
60 | 1.4 | 2.9 | --- | --- | --- | --- | --- |
70 | 1.7 | 3.4 | --- | --- | --- | --- | --- |
80 | 1.9 | 3.8 | --- | --- | --- | --- | --- |
90 | 2.2 | --- | --- | --- | --- | --- | --- |
100 | 2.4 | --- | --- | --- | --- | --- | --- |
Patient weight (kg) | Dose or Drug Delivery Rate (ng/kg/min) | ||||||
4 | 6 | 8 | 10 | 12 | 14 | 16 | |
Infusion Delivery Rate (mL/hr) | |||||||
20 | --- | --- | 1 | 1.2 | 1.4 | 1.7 | 1.9 |
30 | --- | 1.1 | 1.4 | 1.8 | 2.2 | 2.5 | 2.9 |
40 | 1 | 1.4 | 1.9 | 2.4 | 2.9 | 3.4 | 3.8 |
50 | 1.2 | 1.8 | 2.4 | 3 | 3.6 | --- | --- |
60 | 1.4 | 2.2 | 2.9 | 3.6 | --- | --- | --- |
70 | 1.7 | 2.5 | 3.4 | --- | --- | --- | --- |
80 | 1.9 | 2.9 | 3.8 | --- | --- | --- | --- |
90 | 2.2 | 3.2 | --- | --- | --- | --- | --- |
100 | 2.4 | 3.6 | --- | --- | --- | --- | --- |
Patient weight (kg) | Dose or Drug Delivery Rate (ng/kg/min) | ||||||
4 | 6 | 8 | 10 | 12 | 14 | 16 | |
Infusion Delivery Rate (mL/hr) | |||||||
20 | --- | --- | --- | --- | 1 | 1.1 | 1.3 |
30 | --- | --- | 1 | 1.2 | 1.4 | 1.7 | 1.9 |
40 | --- | 1 | 1.3 | 1.6 | 1.9 | 2.2 | 2.6 |
50 | --- | 1.2 | 1.6 | 2 | 2.4 | 2.8 | 3.2 |
60 | 1 | 1.4 | 1.9 | 2.4 | 2.9 | 3.4 | 3.8 |
70 | 1.1 | 1.7 | 2.2 | 2.8 | 3.4 | 3.9 | --- |
80 | 1.3 | 1.9 | 2.6 | 3.2 | 3.8 | --- | --- |
90 | 1.4 | 2.2 | 2.9 | 3.6 | --- | --- | --- |
100 | 1.6 | 2.4 | 3.2 | 4 | --- | --- | --- |
Patient weight (kg) | Dose or Drug Delivery Rate (ng/kg/min) | |||||
6 | 8 | 10 | 12 | 14 | 16 | |
30 | --- | --- | --- | --- | --- | 1 |
40 | --- | --- | --- | 1 | 1.1 | 1.3 |
50 | --- | --- | 1 | 1.2 | 1.4 | 1.6 |
60 | --- | 1 | 1.2 | 1.4 | 1.7 | 1.9 |
70 | --- | 1.1 | 1.4 | 1.7 | 2 | 2.2 |
80 | 1 | 1.3 | 1.6 | 1.9 | 2.2 | 2.6 |
90 | 1.1 | 1.4 | 1.8 | 2.2 | 2.5 | 2.9 |
100 | 1.2 | 1.6 | 2 | 2.4 | 2.8 | 3.2 |
Epoprostenol for Injection
contains epoprostenol sodium equivalent to 0.5 mg (500,000 ng) or 1.5 mg (1,500,000 ng) epoprostenol and is supplied as a sterile lyophilized powder or cake in a 10 mL vial.Limited published data from case series and case reports with epoprostenol have not established a drug associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes when used during pregnancy. There are risks to the mother and fetus from untreated pulmonary arterial hypertension (
Pregnant women with untreated pulmonary arterial hypertension are at risk for heart failure, stroke, preterm delivery, and maternal and fetal death.
Embryo-fetal development studies have been performed in rats and rabbits during organogenesis. Epoprostenol sodium doses up to 100 mcg/kg/day, a dose that was maternally toxic in rabbits but not in rats, (600 mcg/m2/day in rats, 2.5 times the MRHD, and 1,180 mcg/m2/day in rabbits, 4.8 times the MRHD based on body surface area), had no effect on the fetus.
In a postnatal development study, epoprostenol sodium was administered subcutaneously to female rats for 2 weeks prior to mating through weaning and to male rats for 60 days prior to and through mating at a male and female toxic dose of up to 100 mcg/kg/day (600 mcg/m2/day, 2.5 times the MRHD based on body surface area). There was no effect on growth and development of the offspring.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
A large study evaluating the effect of epoprostenol on survival in NYHA Class III and IV patients with congestive heart failure due to severe left ventricular systolic dysfunction was terminated after an interim analysis of 471 patients revealed a higher mortality in patients receiving epoprostenol plus conventional therapy than in those receiving conventional therapy alone. The chronic use of epoprostenol in patients with congestive heart failure due to severe left ventricular systolic dysfunction is therefore contraindicated.
Some patients with pulmonary hypertension have developed pulmonary edema during dose initiation, which may be associated with pulmonary veno-occlusive disease. Epoprostenol should not be used chronically in patients who develop pulmonary edema during dose initiation.
Epoprostenol is also contraindicated in patients with known hypersensitivity to the drug or to structurally related compounds.
• Do not abruptly lower the dose or withdraw dosing. All dosing initiation and changes should be closely monitored. (,5.2 Chronic Use and Dose AdjustmentDuring chronic use, deliver epoprostenol continuously on an ambulatory basis through a permanent indwelling central venous catheter. Unless contraindicated, administer anticoagulant therapy to patients receiving epoprostenol to reduce the risk of pulmonary thromboembolism or systemic embolism through a patent foramen ovale. To reduce the risk of infection, use aseptic technique in the reconstitution and administration of epoprostenol and in routine catheter care. Because epoprostenol is metabolized rapidly, even brief interruptions in the delivery of epoprostenol may result in symptoms associated with rebound pulmonary hypertension including dyspnea, dizziness, and asthenia. Intravenous therapy with epoprostenol will likely be needed for prolonged periods, possibly years, so consider the patient’s capacity to accept and care for a permanent intravenous catheter and infusion pump.
Based on clinical trials, the acute hemodynamic response (reduction in pulmonary artery resistance) to epoprostenol did not correlate well with improvement in exercise tolerance or survival during chronic use of epoprostenol. Adjust dosage of epoprostenol during chronic use at the first sign of recurrence or worsening of symptoms attributable to pulmonary hypertension or the occurrence of adverse events associated with epoprostenol
[see Dosage and Administration (2.2)]. Following dosage adjustments, monitor standing and supine blood pressure and heart rate closely for several hours.)5.3 Withdrawal EffectsAbrupt withdrawal (including interruptions in drug delivery) or sudden large reductions in dosage of epoprostenol may result in symptoms associated with rebound pulmonary hypertension, including dyspnea, dizziness, and asthenia. In clinical trials, one Class III primary pulmonary hypertension patient’s death was judged attributable to the interruption of epoprostenol. Avoid abrupt withdrawal.