Erythrocin Lactobionate
(Erythromycin Lactobionate)Erythrocin Lactobionate Prescribing Information
Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) is indicated in the treatment of infections caused by susceptible strains of the designated organisms in the diseases listed below when oral administration is not possible or when the severity of the infection requires immediate high serum levels of erythromycin. Intravenous therapy should be replaced by oral administration at the appropriate time.
Upper respiratory tract infections of mild to moderate degree caused by
Lower respiratory tract infections of mild to moderate severity caused by
Respiratory tract infections due to
Skin and skin structure infections of mild to moderate severity caused by
Diphtheria: As an adjunct to antitoxin infections due to
Erythrasma: In the treatment of infections due to
Acute pelvic inflammatory disease caused by
Before treatment of gonorrhea, patients who are suspected of also having syphilis should have a microscopic examination for
Legionnaires' Disease caused by
Penicillin is considered by the American Heart Association to be the drug of choice in the prevention of initial attacks of rheumatic fever (treatment of Group A beta-hemolytic streptococcal infections of the upper respiratory tract e.g., tonsillitis, or pharyngitis).1 Erythromycin is indicated for the treatment of penicillin-allergic patients. The therapeutic dose should be administered for ten days.
Penicillin or sulfonamides are considered by the American Heart Association to be the drugs of choice in the prevention of recurrent attacks of rheumatic fever. In patients who are allergic to penicillin and sulfonamides, oral erythromycin is recommended by the American Heart Association in the long-term prophylaxis of streptococcal pharyngitis (for the prevention of recurrent attacks of rheumatic fever).1
Although no controlled clinical efficacy trials have been conducted, oral erythromycin has been recommended by the American Heart Association for prevention of bacterial endocarditis in penicillin-allergic patients with prosthetic cardiac valves, most congenital cardiac malformations, surgically constructed systemic pulmonary shunts, rheumatic or other acquired valvular dysfunction, idiopathic hypertrophic subaortic stenosis (IHSS), previous history of bacterial endocarditis and mitral valve prolapse with insufficiency when they undergo dental procedures and surgical procedures of the upper respiratory tract.2
To reduce the development of drug-resistant bacteria and maintain the effectiveness of erythromycin and other antibacterial drugs, erythromycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
For the treatment of severe infections in adults and pediatric patients, the recommended intravenous dose of erythromycin lactobionate is 15 to 20 mg/kg/day. Higher doses, up to 4 g/day, may be given for severe infections.
Administration of doses of ≥4 g/day may increase the risk for the development of erythromycin-induced hearing loss in elderly patients, particularly those with reduced renal or hepatic function. Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) in the ADD-Vantage system must be administered by intermittent intravenous infusion only. Due to the irritative properties of erythromycin, IV push is an unacceptable route of administration.
Intravenous erythromycin should be replaced by oral erythromycin as soon as possible.
For intermittent infusion: administer one-fourth the total daily dose of erythromycin lactobionate by intravenous infusion in 20 to 60 minutes at intervals not greater than every six hours. The final diluted solution of erythromycin lactobionate is prepared to give a concentration of 1 to 5 mg/mL. No less than 100 mL of IV diluent should be used. Infusion should be sufficiently slow to minimize pain along the vein.
For treatment of acute pelvic inflammatory disease caused by
For treatment of Legionnaires' Disease: Although optimal doses have not been established, doses utilized in reported clinical data were 1 to 4 grams daily in divided doses.
Administration of doses of ≥4 g/day may increase the risk for the development of erythromycin-induced hearing loss in elderly patients, particularly those with reduced renal or hepatic function.
In the treatment of Group A beta-hemolytic streptococcal infections of the upper respiratory tract (e.g., tonsillitis or pharyngitis), the therapeutic dosage of erythromycin should be administered for ten days. The American Heart Association suggests a dosage of 250 mg of erythromycin orally, twice a day in long-term prophylaxis of streptococcal upper respiratory tract infections for the prevention of recurring attacks of rheumatic fever in patients allergic to penicillin and sulfonamides.1
In prophylaxis against bacterial endocarditis (See
Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) is indicated in the treatment of infections caused by susceptible strains of the designated organisms in the diseases listed below when oral administration is not possible or when the severity of the infection requires immediate high serum levels of erythromycin. Intravenous therapy should be replaced by oral administration at the appropriate time.
Upper respiratory tract infections of mild to moderate degree caused by
Lower respiratory tract infections of mild to moderate severity caused by
Respiratory tract infections due to
Skin and skin structure infections of mild to moderate severity caused by
Diphtheria: As an adjunct to antitoxin infections due to
Erythrasma: In the treatment of infections due to
Acute pelvic inflammatory disease caused by
Before treatment of gonorrhea, patients who are suspected of also having syphilis should have a microscopic examination for
Legionnaires' Disease caused by
Penicillin is considered by the American Heart Association to be the drug of choice in the prevention of initial attacks of rheumatic fever (treatment of Group A beta-hemolytic streptococcal infections of the upper respiratory tract e.g., tonsillitis, or pharyngitis).1Erythromycin is indicated for the treatment of penicillin-allergic patients. The therapeutic dose should be administered for ten days.
Penicillin or sulfonamides are considered by the American Heart Association to be the drugs of choice in the prevention of recurrent attacks of rheumatic fever. In patients who are allergic to penicillin and sulfonamides, oral erythromycin is recommended by the American Heart Association in the long-term prophylaxis of streptococcal pharyngitis (for the prevention of recurrent attacks of rheumatic fever).1
Although no controlled clinical efficacy trials have been conducted, oral erythromycin has been recommended by the American Heart Association for prevention of bacterial endocarditis in penicillin-allergic patients with prosthetic cardiac valves, most congenital cardiac malformations, surgically constructed systemic pulmonary shunts, rheumatic or other acquired valvular dysfunction, idiopathic hypertrophic subaortic stenosis (IHSS), previous history of bacterial endocarditis and mitral valve prolapse with insufficiency when they undergo dental procedures and surgical procedures of the upper respiratory tract.2
To reduce the development of drug-resistant bacteria and maintain the effectiveness of erythromycin and other antibacterial drugs, erythromycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
The Erythrocin Lactobionate-IV ADD-Vantage vial may be used with either 0.9% Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP in the ADD-Vantage flexible diluent container. The 500 mg ADD-Vantage vials must be used as single doses with the 100 mL ADD-Vantage flexible diluent containers. The resulting solution will contain erythromycin activity equal to approximately 5 mg/mL.
Peel overwrap at corner and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
1 Remove the protective covers from the top of the vial and the vial port on the diluent container as follows:a. To remove the breakaway vial cap, swing the pull ring over the top of the vial and pull down far enough to start the opening (See FIGURE 1.), then pull straight up to remove the cap. (See FIGURE 2.)NOTE:Do not access vial with syringe.
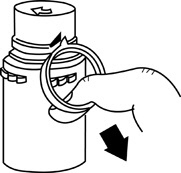
o To remove the vial port cover, grasp the tab on the pull ring, pull up to break the three tie strings, then pull back to remove the cover. (See FIGURE 3.)
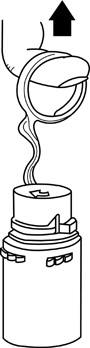
2 Screw the vial into the vial port until it will go no further. THE VIAL MUST BE SCREWED IN TIGHTLY TO ASSURE A SEAL. This occurs approximately 1/2 turn (180°) after the first audible click. (See FIGURE 4.) The clicking sound does not assure a seal; the vial must be turned as far as it will go.NOTE:Once vial is seated, do not attempt to remove. (See FIGURE 4.)
4 Label appropriately.
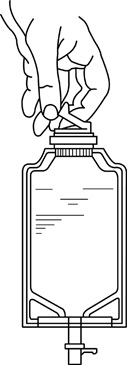
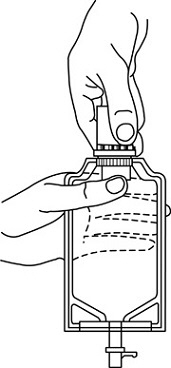
• Squeeze the bottom of the diluent container gently to inflate the portion of the container surrounding the end of the drug vial.• With the other hand, push the drug vial down into the container telescoping the walls of the container. Grasp the inner cap of the vial through the walls of the container. (See FIGURE 5.)• Pull the inner cap from the drug vial. (See FIGURE 6.) Verify that the rubber stopper has been pulled out, allowing the drug and diluent to mix.• Mix container contents thoroughly and use within the specified time.
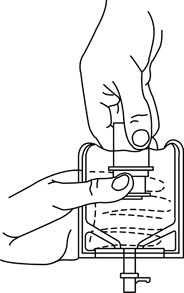
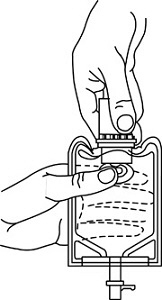
1 Confirm the activation and admixture of vial contents.2 Check for leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired.3 Close flow control clamp of administration set.4 Remove cover from outlet port at bottom of container.5 Insert piercing pin of administration set into port with a twisting motion until the pin is firmly seated.
6 Lift the free end of the hanger loop on the bottom of the vial, breaking the two tie strings. Bend the loop outward to lock it in the upright position, then suspend container from hanger.7 Squeeze and release drip chamber to establish proper fluid level in chamber.8 Open flow control clamp and clear air from set. Close clamp.9 Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.10 Regulate rate of administration with flow control clamp.
The final diluted solution of erythromycin lactobionate should be completely administered
The final diluted solution of erythromycin lactobionate should be completely administered
No drug or chemical agent should be added to an Erythrocin Lactobionate-IV fluid admixture unless its effect on the chemical and physical stability of the solution has first been determined.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Erythromycin is contraindicated in patients with known hypersensitivity to this antibiotic. Erythromycin is contraindicated in patients taking terfenadine or astemizole, cisapride, pimozide, ergotamine, or dihydroergotamine (See
Do not use erythromycin concomitantly with 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA) reductase inhibitors (statins) that are extensively metabolized by cytochrome P450 isoform 3A4 (lovastatin or simvastatin), due to the increased risk of myopathy, including rhabdomyolysis (See
Erythromycin has been associated with QT prolongation and ventricular arrhythmias, including ventricular tachycardia and torsades de pointes. (See
There have been reports of hepatic dysfunction, with or without jaundice occurring in patients receiving oral erythromycin products. Since erythromycin is principally excreted by the liver, monitor for liver toxicity when erythromycin is administered to patients with impaired hepatic function (See
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against
Life-threatening episodes of ventricular tachycardia associated with prolonged QT interval (torsades de pointes) have been reported in some patients after intravenous administration of erythromycin lactobionate.
Susceptibility to the development of torsades de pointes arrhythmias, a rare but serious cardiac condition, is related to electrolyte imbalance, hepatic dysfunction, myocardial ischemia, left ventricular dysfunction, idiopathic Q-T prolongation, and concurrent antiarrhythmic therapy.3Elderly patients exhibit a greater frequency of decreased hepatic function, cardiac function, and of concomitant disease and other drug therapy, and therefore should be monitored carefully during ErythrocinTMtherapy.
There have been reports of IHPS occurring in infants following erythromycin therapy. Since erythromycin may be used in the treatment of conditions in infants which are associated with significant mortality or morbidity (such as pertussis or chlamydia), the benefit of erythromycin therapy needs to be weighed against the potential risk of developing IHPS. Parents or caregivers of infants receiving erythromycin should be informed to contact their physician if vomiting or irritability with feeding occurs.
Serious adverse reactions have been reported in patients taking erythromycin concomitantly with CYP3A4 substrates. These include colchicine toxicity with colchicine; rhabdomyolysis with simvastatin, lovastatin, and atorvastatin; and hypotension with calcium channel blockers metabolized by CYP3A4 (e.g. verapamil, amlodipine, diltiazem, vasospasm and ischemia with ergotamine/dihydroergotamine) (See
Side effects following the use of intravenous erythromycin are rare. Occasional venous irritation has been encountered, but if the infusion is given slowly, in dilute solution, preferably by continuous intravenous infusion or intermittent infusion in no less than 20 to 60 minutes, pain and vessel trauma are minimized.
Allergic reactions ranging from urticaria to anaphylaxis have occurred. Skin reactions ranging from mild eruptions to erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis have been reported rarely.
There have been isolated reports of reversible hearing loss occurring chiefly in patients with renal insufficiency and in patients receiving high doses of erythromycin.
Elderly patients, particularly those with reduced renal or hepatic function, may also be at increased risk for developing this effect when ErythrocinTM doses of 4 grams/day or higher are given (See
For the treatment of severe infections in adults and pediatric patients, the recommended intravenous dose of erythromycin lactobionate is 15 to 20 mg/kg/day. Higher doses, up to 4 g/day, may be given for severe infections.
Administration of doses of ≥4 g/day may increase the risk for the development of erythromycin-induced hearing loss in elderly patients, particularly those with reduced renal or hepatic function. Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) in the ADD-Vantage system must be administered by intermittent intravenous infusion only. Due to the irritative properties of erythromycin, IV push is an unacceptable route of administration.
Intravenous erythromycin should be replaced by oral erythromycin as soon as possible.
For intermittent infusion: administer one-fourth the total daily dose of erythromycin lactobionate by intravenous infusion in 20 to 60 minutes at intervals not greater than every six hours. The final diluted solution of erythromycin lactobionate is prepared to give a concentration of 1 to 5 mg/mL. No less than 100 mL of IV diluent should be used. Infusion should be sufficiently slow to minimize pain along the vein.
For treatment of acute pelvic inflammatory disease caused by
For treatment of Legionnaires' Disease: Although optimal doses have not been established, doses utilized in reported clinical data were 1 to 4 grams daily in divided doses.
Administration of doses of ≥4 g/day may increase the risk for the development of erythromycin-induced hearing loss in elderly patients, particularly those with reduced renal or hepatic function.
In the treatment of Group A beta-hemolytic streptococcal infections of the upper respiratory tract (e.g., tonsillitis or pharyngitis), the therapeutic dosage of erythromycin should be administered for ten days. The American Heart Association suggests a dosage of 250 mg of erythromycin orally, twice a day in long-term prophylaxis of streptococcal upper respiratory tract infections for the prevention of recurring attacks of rheumatic fever in patients allergic to penicillin and sulfonamides.1
In prophylaxis against bacterial endocarditis (See
The Erythrocin Lactobionate-IV ADD-Vantage vial may be used with either 0.9% Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP in the ADD-Vantage flexible diluent container. The 500 mg ADD-Vantage vials must be used as single doses with the 100 mL ADD-Vantage flexible diluent containers. The resulting solution will contain erythromycin activity equal to approximately 5 mg/mL.
Peel overwrap at corner and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
1 Remove the protective covers from the top of the vial and the vial port on the diluent container as follows:a. To remove the breakaway vial cap, swing the pull ring over the top of the vial and pull down far enough to start the opening (See FIGURE 1.), then pull straight up to remove the cap. (See FIGURE 2.)NOTE:Do not access vial with syringe.


o To remove the vial port cover, grasp the tab on the pull ring, pull up to break the three tie strings, then pull back to remove the cover. (See FIGURE 3.)
2 Screw the vial into the vial port until it will go no further. THE VIAL MUST BE SCREWED IN TIGHTLY TO ASSURE A SEAL. This occurs approximately 1/2 turn (180°) after the first audible click. (See FIGURE 4.) The clicking sound does not assure a seal; the vial must be turned as far as it will go.NOTE:Once vial is seated, do not attempt to remove. (See FIGURE 4.)


3 Recheck the vial to assure that it is tight by trying to turn it further in the direction of assembly. 4 Label appropriately.
• Squeeze the bottom of the diluent container gently to inflate the portion of the container surrounding the end of the drug vial.• With the other hand, push the drug vial down into the container telescoping the walls of the container. Grasp the inner cap of the vial through the walls of the container. (See FIGURE 5.)• Pull the inner cap from the drug vial. (See FIGURE 6.) Verify that the rubber stopper has been pulled out, allowing the drug and diluent to mix.• Mix container contents thoroughly and use within the specified time.


1 Confirm the activation and admixture of vial contents.2 Check for leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired.3 Close flow control clamp of administration set.4 Remove cover from outlet port at bottom of container.5 Insert piercing pin of administration set into port with a twisting motion until the pin is firmly seated.
6 Lift the free end of the hanger loop on the bottom of the vial, breaking the two tie strings. Bend the loop outward to lock it in the upright position, then suspend container from hanger.7 Squeeze and release drip chamber to establish proper fluid level in chamber.8 Open flow control clamp and clear air from set. Close clamp.9 Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.10 Regulate rate of administration with flow control clamp.
The final diluted solution of erythromycin lactobionate should be completely administered
The final diluted solution of erythromycin lactobionate should be completely administered
No drug or chemical agent should be added to an Erythrocin Lactobionate-IV fluid admixture unless its effect on the chemical and physical stability of the solution has first been determined.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.






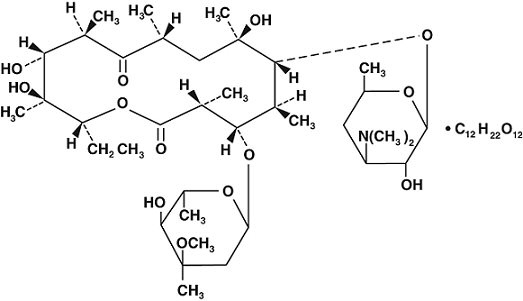
ErythrocinTM Lactobionate-IV (erythromycin lactobionate for injection, USP) is a lyophilized powder for intravenous infusion only. It is available as a sterile, white to off-white lyophilized powder in single dose ADD-VantageTM vials. Each vial contains the equivalent of 500 mg of erythromycin activity. It is prepared as a solution and lyophilized in its final container. The lactobionic acid content is 244 mg per vial. Lactobionic acid and/or erythromycin is used to adjust the pH during the manufacture of the product. The Erythrocin Lactobionate-IV ADD-VantageTM vial is designed for use only with the ADD-Vantage flexible diluent container. After appropriate dilution, the Erythrocin Lactobionate-IV ADD-Vantage Delivery System contains erythromycin lactobionate equivalent to 500 mg of erythromycin activity in 100 mL. The pH of the reconstituted solution is 6.5 - 7.5.
The solutions contain no bacteriostat, antimicrobial agent (except erythromycin) or buffer and are intended for use as a single-dose injection only with the ADD-Vantage flexible diluent container.
Erythromycin is produced by a strain of
Erythromycin diffuses readily into most body fluids. In the absence of meningeal inflammation, low concentrations are normally achieved in the spinal fluid but the passage of the drug across the blood-brain barrier increases in meningitis. Erythromycin crosses the placental barrier and is excreted in breast milk. Erythromycin is not removed by peritoneal dialysis or hemodialysis.
In the presence of normal hepatic function, erythromycin is concentrated in the liver and is excreted in the bile; the effect of hepatic dysfunction on biliary excretion of erythromycin is not known. From 12 to 15 percent of intravenously administered erythromycin is excreted in active form in the urine.
Intravenous infusion of 500 mg of erythromycin lactobionate at a constant rate over 1 hour in fasting adults produced a mean serum erythromycin level of approximately 7 mcg/mL at 20 minutes, 10 mcg/mL at 1 hour, 2.6 mcg/mL at 2.5 hours, and 1 mcg/mL at 6 hours.
Erythromycin acts by inhibition of protein synthesis by binding 50 S ribosomal subunits of susceptible organisms. It does not affect nucleic acid synthesis.
Resistance to erythromycin in
Antagonism has been demonstrated
Erythromycin has been shown to be active against most isolates of the following microorganisms, both
Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) is indicated in the treatment of infections caused by susceptible strains of the designated organisms in the diseases listed below when oral administration is not possible or when the severity of the infection requires immediate high serum levels of erythromycin. Intravenous therapy should be replaced by oral administration at the appropriate time.
Upper respiratory tract infections of mild to moderate degree caused by
Lower respiratory tract infections of mild to moderate severity caused by
Respiratory tract infections due to
Skin and skin structure infections of mild to moderate severity caused by
Diphtheria: As an adjunct to antitoxin infections due to
Erythrasma: In the treatment of infections due to
Acute pelvic inflammatory disease caused by
Before treatment of gonorrhea, patients who are suspected of also having syphilis should have a microscopic examination for
Legionnaires' Disease caused by
Penicillin is considered by the American Heart Association to be the drug of choice in the prevention of initial attacks of rheumatic fever (treatment of Group A beta-hemolytic streptococcal infections of the upper respiratory tract e.g., tonsillitis, or pharyngitis).1Erythromycin is indicated for the treatment of penicillin-allergic patients. The therapeutic dose should be administered for ten days.
Penicillin or sulfonamides are considered by the American Heart Association to be the drugs of choice in the prevention of recurrent attacks of rheumatic fever. In patients who are allergic to penicillin and sulfonamides, oral erythromycin is recommended by the American Heart Association in the long-term prophylaxis of streptococcal pharyngitis (for the prevention of recurrent attacks of rheumatic fever).1
Although no controlled clinical efficacy trials have been conducted, oral erythromycin has been recommended by the American Heart Association for prevention of bacterial endocarditis in penicillin-allergic patients with prosthetic cardiac valves, most congenital cardiac malformations, surgically constructed systemic pulmonary shunts, rheumatic or other acquired valvular dysfunction, idiopathic hypertrophic subaortic stenosis (IHSS), previous history of bacterial endocarditis and mitral valve prolapse with insufficiency when they undergo dental procedures and surgical procedures of the upper respiratory tract.2
To reduce the development of drug-resistant bacteria and maintain the effectiveness of erythromycin and other antibacterial drugs, erythromycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Aerobic bacteria
Gram-positive bacteria
- Corynebacterium diphtheriae
- Corynebacterium minutissimum
- Staphylococcus aureus
- Streptococcus pneumoniae
- Streptococcus pyogenes
Gram-negative bacteria
- Legionella pneumophila
- Neisseria gonorrhoeae
Other Microorganisms
- Mycoplasma pneumoniae
The following
Aerobic bacteria
Gram-negative bacteria
- Moraxella catarrhalis
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: