Ethacrynic Acid
Ethacrynic Acid Prescribing Information
Ethacrynic acid tablets are indicated for treatment of edema when an agent with greater diuretic potential than those commonly employed is required.
1. Treatment of the edema associated with congestive heart failure, cirrhosis of the liver, and renal disease, including the nephrotic syndrome.
2. Short-term management of ascites due to malignancy, idiopathic edema, and lymphedema.
3. Short-term management of hospitalized pediatric patients, other than infants, with congenital heart disease or the nephrotic syndrome.
Intravenous ethacrynate sodium is indicated when a rapid onset of diuresis is desired, e.g., in acute pulmonary edema, or when gastrointestinal absorption is impaired or oral medication is not practicable.
Ethacrynic acid tablets are available for oral use as 25 mg tablets.
The patient should be weighed under standard conditions before and during the institution of diuretic therapy with this compound. Small alterations in dose should effectively prevent a massive diuretic response. The following schedule may be helpful in determining the smallest effective dose.
Day 1 — 50 mg once daily after a meal
Day 2 — 50 mg twice daily after meals, if necessary
Day 3 — 100 mg in the morning and 50 to 100 mg following the afternoon or evening meal, depending upon response to the morning dose.
A few patients may require initial and maintenance doses as high as 200 mg twice daily. These higher doses, which should be achieved gradually, are most often required in patients with severe, refractory edema.
It is usually possible to reduce the dosage and frequency of administration once dry weight has been achieved.
The chloruretic effect of this agent may give rise to retention of bicarbonate and a metabolic alkalosis. This may be corrected by giving chloride (ammonium chloride or arginine chloride). Ammonium chloride should not be given to cirrhotic patients.
Ethacrynic acid tablets, has additive effects when used with other diuretics. For example, a patient who is on maintenance dosage of an oral diuretic may require additional intermittent diuretic therapy, such as an organomercurial, for the maintenance of basal weight. The intermittent use of ethacrynic acid tablets orally may eliminate the need for injections of organomercurials. Small doses of ethacrynic acid tablets may be added to existing diuretic regimens to maintain basal weight. This drug may potentiate the action of carbonic anhydrase inhibitors, with augmentation of natriuresis and kaliuresis. Therefore, when adding ethacrynic acid tablets the initial dose and changes of dose should be in 25 mg increments, to avoid electrolyte depletion. Rarely, patients who failed to respond to ethacrynic acid have responded to older established agents.
While many patients do not require supplemental potassium, the use of potassium chloride or potassium-sparing agents, or both, during treatment with ethacrynic acid tablets is advisable, especially in cirrhotic or nephrotic patients and in patients receiving digitalis.
Salt liberalization usually prevents the development of hyponatremia and hypochloremia. During treatment with ethacrynic acid tablets, salt may be liberalized to a greater extent than with other diuretics. Cirrhotic patients, however, usually require at least moderate salt restriction concomitant with diuretic therapy.
Intravenous ethacrynate sodium is for intravenous use when oral intake is impractical or in urgent conditions, such as acute pulmonary edema.
All diuretics, including ethacrynic acid, are contraindicated in anuria. If increasing electrolyte imbalance, azotemia, and/or oliguria occur during treatment of severe, progressive renal disease, the diuretic should be discontinued.
In a few patients this diuretic has produced severe, watery diarrhea. If this occurs, it should be discontinued and not used again.
Until further experience in infants is accumulated, therapy with oral ethacrynic acid is contraindicated.
Hypersensitivity to any component of this product.
Anorexia, malaise, abdominal discomfort or pain, dysphagia, nausea, vomiting, and diarrhea have occurred. These are more frequent with large doses or after one to three months of continuous therapy. A few patients have had sudden onset of profuse, watery diarrhea. Discontinue ethacrynic acid if diarrhea is severe and do not give it again. Gastrointestinal bleeding has occurred in some patients. Rarely, acute pancreatitis has been reported.
Reversible hyperuricemia and acute gout have been reported. Acute symptomatic hypoglycemia with convulsions occurred in two uremic patients who received doses above those recommended. Hyperglycemia has been reported. Rarely, jaundice and abnormal liver function tests have been reported in seriously ill patients
receiving multiple drug therapy, including ethacrynic acid.
Agranulocytosis or severe neutropenia has been reported in a few critically ill patients also receiving agents known to produce this effect. Thrombocytopenia has been reported rarely. Henoch-Schönlein purpura has been reported rarely in patients with rheumatic heart disease receiving multiple drug therapy, including ethacrynic acid.
(see
Deafness, tinnitus and vertigo with a sense of fullness in the ears, and blurred vision have occurred.
Headache, fatigue, apprehension, confusion.
Skin rash, fever, chills, hematuria.
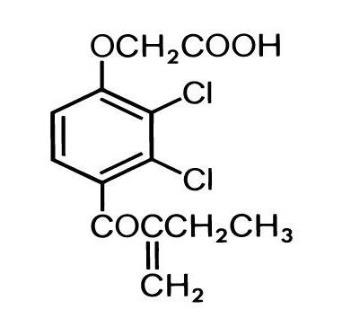
Ethacrynic acid is an unsaturated ketone derivative of an aryloxyacetic acid. It is designated chemically as [2, 3-dichloro-4-(2-methylene-1-oxobutyl) phenoxy] acetic acid, and has a molecular weight of 303.14. Ethacrynic acid, USP is a white, or practically white, crystalline powder, very slightly soluble in water, but soluble in most organic solvents such as alcohols, chloroform, and benzene. Its empirical formula is C13H12Cl2O4 and its structural formula is:
Ethacrynic acid tablets, USP are supplied as 25 mg tablets for oral use. The tablets contain the following inactive ingredients: lactose monohydrate, magnesium stearate, pregelatinized starch (corn) and talc.
Ethacrynic acid acts on the ascending limb of the loop of Henle and on the proximal and distal tubules. Urinary output is usually dose dependent and related to the magnitude of fluid accumulation. Water and electrolyte excretion may be increased several times over that observed with thiazide diuretics, since ethacrynic acid inhibits reabsorption of a much greater proportion of filtered sodium than most other diuretic agents. Therefore, ethacrynic acid is effective in many patients who have significant degrees of renal insufficiency (see
The electrolyte excretion pattern of ethacrynic acid varies from that of the thiazides and mercurial diuretics. Initial sodium and chloride excretion is usually substantial and chloride loss exceeds that of sodium. With prolonged administration, chloride excretion declines, and potassium and hydrogen ion excretion may increase. Ethacrynic acid is effective whether or not there is clinical acidosis or alkalosis.
Although ethacrynic acid, in carefully controlled studies in animals and experimental subjects, produces a more favorable sodium/potassium excretion ratio than the thiazides, in patients with increased diuresis excessive amounts of potassium may be excreted.
Onset of action is rapid, usually within 30 minutes after an oral dose of ethacrynic acid. After oral use, diuresis peaks in about 2 hours and lasts about 6 to 8 hours.
The sulfhydryl binding propensity of ethacrynic acid differs somewhat from that of the organomercurials. Its mode of action is not by carbonic anhydrase inhibition.
Ethacrynic acid does not cross the blood-brain barrier.