Ethosuximide
Ethosuximide Prescribing Information
Ethosuximide is indicated for the control of absence (petit mal) epilepsy.
Ethosuximide is administered by the oral route. The
Ethosuximide may be administered in combination with other anticonvulsants when other forms of epilepsy coexist with absence (petit mal). The
Ethosuximide should not be used in patients with a history of hypersensitivity to succinimides.
Allergic reaction, Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS).
Since ethosuximide may interact with concurrently administered antiepileptic drugs, periodic serum level determinations of these drugs may be necessary (e.g., ethosuximide may elevate phenytoin serum levels and valproic acid has been reported to both increase and decrease ethosuximide levels).
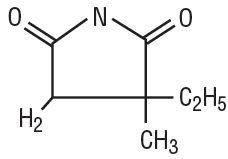
Ethosuximide is an anticonvulsant succinimide, chemically designated as alpha-ethyl-alphamethyl-succinimide, with the following structural formula:
Each ethosuximide capsule contains 250 mg ethosuximide, USP. In addition, each capsule contains FD&C Red No. 3, FD&C Yellow No. 6, Gelatin, NF, Glycerin, USP, Polyethylene Glycol 400, NF, Purified Water. The white ink contains N-butyl Alcohol, Propylene Glycol, Shellac Glaze, and Titanium Dioxide.