Etoposide Prescribing Information
Etoposide should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Severe myelosuppression with resulting infection or bleeding may occur.
Etoposide Injection, USP is indicated in the management of the following neoplasms:
Etoposide is contraindicated in patients who have demonstrated a previous hypersensitivity to etoposide or any component of the formulation.
The following data on adverse reactions are based on intravenous administration of etoposide as a single agent, using several different dose schedules for treatment of a wide variety of malignancies.
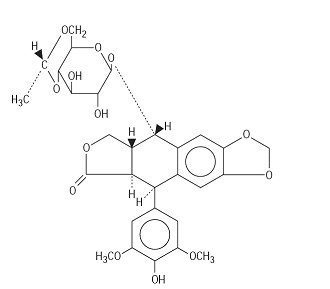
Etoposide (also commonly known as VP-16) is a semisynthetic derivative of podophyllotoxin used in the treatment of certain neoplastic diseases. It is 4'-demethylepipodophyllotoxin 9-[4,6-0-(R)-ethylidene-β-D-glucopyranoside]. It is very soluble in methanol and chloroform, slightly soluble in ethanol and sparingly soluble in water and ether. It is made more miscible with water by means of organic solvents. It has a molecular weight of 588.58 and a molecular formula of C29H32O13.
Etoposide Injection, USP is available for intravenous use as 20 mg/mL solution in 100 mg (5 mL), 500 mg (25 mL), and 1 g (50 mL) sterile, multiple-dose vials. The pH of the clear, nearly colorless to yellow liquid is 3 to 4. Each mL contains 20 mg etoposide USP, 2 mg citric acid, 30 mg benzyl alcohol, 80 mg modified polysorbate 80/tween 80, 650 mg polyethylene glycol 300, and 30.5 percent (v/v) alcohol.
The structural formula is: