Ezetimibe And Simvastatin
Ezetimibe And Simvastatin Prescribing Information
Therapy with lipid-altering agents should be only one component of multiple risk factor intervention in individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia.
Drug therapy is indicated as an adjunct to diet when the response to a diet restricted in saturated fat and cholesterol and other nonpharmacologic measures alone has been inadequate.
- Ezetimibe and simvastatin tablets 10/10 mg, (ezetimibe, USP 10 mg and simvastatin, USP 10 mg tablets) are white to off-white capsule shaped tablets debossed with “AA 70” on one side and plain on the other side.
- Ezetimibe and simvastatin tablets 10/20 mg, (ezetimibe, USP 10 mg and simvastatin, USP 20 mg tablets) are white to off-white capsule shaped tablets debossed with “AA 71” on one side and plain on the other side.
- Ezetimibe and simvastatin tablets 10/40 mg, (ezetimibe, USP 10 mg and simvastatin, USP 40 mg tablets) are white to off-white capsule shaped tablets debossed with “AA 72” on one side and plain on the other side.
- Ezetimibe and simvastatin tablets 10/80 mg, (ezetimibe, USP 10 mg and simvastatin, USP 80 mg tablets) are white to off-white capsule shaped tablets debossed with “AA 73” on one side and plain on the other side.
Ezetimibe and simvastatin tablets are contraindicated in the following conditions:
- Concomitant administration of strong CYP3A4 inhibitors (e.g., itraconazole, ketoconazole, posaconazole, voriconazole, HIV protease inhibitors, boceprevir, telaprevir, erythromycin, clarithromycin, telithromycin, nefazodone, and cobicistat-containing products) [see.]
5.1 Myopathy/RhabdomyolysisSimvastatin occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase above ten times the upper limit of normal (ULN). Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. The risk of myopathy is increased by elevated plasma levels of simvastatin and simvastatin acid. Predisposing factors for myopathy include advanced age (≥65 years), female gender, uncontrolled hypothyroidism, and renal impairment. Chinese patients may be at increased risk for myopathy
[see Use in Specific Populations (8.8)].The risk of myopathy, including rhabdomyolysis, is dose related.In a clinical trial database in which 41,413 patients were treated with simvastatin, 24,747 (approximately 60%) of whom were enrolled in studies with a median follow-up of at least 4 years, the incidence of myopathy was approximately 0.03% and 0.08% at 20 and 40 mg/day, respectively. The incidence of myopathy with 80 mg (0.61%) was disproportionately higher than that observed at the lower doses. In these trials, patients were carefully monitored and some interacting medicinal products were excluded.In a clinical trial in which 12,064 patients with a history of myocardial infarction were treated with simvastatin (mean follow-up 6.7 years), the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum creatine kinase [CK] >10 times upper limit of normal [ULN]) in patients on 80 mg/day was approximately 0.9% compared with 0.02% for patients on 20 mg/day. The incidence of rhabdomyolysis (defined as myopathy with a CK >40 times ULN) in patients on 80 mg/day was approximately 0.4% compared with 0% for patients on 20 mg/day. The incidence of myopathy, including rhabdomyolysis, was highest during the first year and then notably decreased during the subsequent years of treatment. In this trial, patients were carefully monitored and some interacting medicinal products were excluded.
The risk of myopathy, including rhabdomyolysis, is greater in patients on simvastatin 80 mg compared with other statin therapies with similar or greater LDL-C-lowering efficacy and compared with lower doses of simvastatin. Therefore, the 10/80-mg dose of ezetimibe and simvastatinshould be used only in patients who have been taking ezetimibe and simvastatin10/80 mg chronically (e.g., for 12 months or more) without evidence of muscle toxicity[see Dosage and Administration, Restricted Dosing for 10/80 mg ]. If, however, a patient who is currently tolerating the 10/80-mg dose of ezetimibe and simvastatinneeds to be initiated on an interacting drug that is contraindicated or is associated with a dose cap for simvastatin, that patient should be switched to an alternative statin or statin-based regimen with less potential for the drug-drug interaction. Patients should be advised of the increased risk of myopathy, including rhabdomyolysis, and to report promptly any unexplained muscle pain, tenderness or weakness. If symptoms occur, treatment should be discontinued immediately[see Warnings and Precautions (5.2)].In the Study of Heart and Renal Protection (SHARP), 9,270 patients with chronic kidney disease were allocated to receive ezetimibe and simvastatin 10/20 mg daily (n=4,650) or placebo (n=4,620). During a median follow-up period of 4.9 years, the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum creatine kinase [CK] >10 times upper limit of normal [ULN]) was 0.2% for ezetimibe and simvastatin and 0.1% for placebo: the incidence of rhabdomyolysis (defined as myopathy with a CK > 40 times ULN) was 0.09% for ezetimibe and simvastatin and 0.02% for placebo.
In postmarketing experience with ezetimibe, cases of myopathy and rhabdomyolysis have been reported. Most patients who developed rhabdomyolysis were taking a statin prior to initiating ezetimibe. However, rhabdomyolysis has been reported with ezetimibe monotherapy and with the addition of ezetimibe to agents known to be associated with increased risk of rhabdomyolysis, such as fibric acid derivatives. Ezetimibe and simvastatin and a fenofibrate, if taking concomitantly, should both be immediately discontinued if myopathy is diagnosed or suspected.
All patients starting therapy with ezetimibe and simvastatinor whose dose of ezetimibe and simvastatinis being increased should be advised of the risk of myopathy, including rhabdomyolysis, and told to report promptly any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuingezetimibe and simvastatin. Ezetimibe and simvastatintherapy should be discontinued immediately if myopathy is diagnosed or suspected.In most cases, muscle symptoms and CK increases resolved when simvastatin treatment was promptly discontinued. Periodic CK determinations may be considered in patients starting therapy with ezetimibe and simvastatin or whose dose is being increased, but there is no assurance that such monitoring will prevent myopathy.Many of the patients who have developed rhabdomyolysis on therapy with simvastatin have had complicated medical histories, including renal insufficiency usually as a consequence of long-standing diabetes mellitus. Such patients taking ezetimibe and simvastatin merit closer monitoring.
Ezetimibe and simvastatin therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Ezetimibe and simvastatin therapy should also be temporarily withheld in any patient experiencing an acute or serious condition predisposing to the development of renal failure secondary to rhabdomyolysis, e.g., sepsis; hypotension; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy.
Drug InteractionsThe risk of myopathy and rhabdomyolysis is increased by elevated plasma levels of simvastatin and simvastatin acid. Simvastatin is metabolized by the cytochrome P450 isoform 3A4. Certain drugs that inhibit this metabolic pathway can raise the plasma levels of simvastatin and may increase the risk of myopathy. These include itraconazole, ketoconazole, posaconazole, and voriconazole, the macrolide antibiotics erythromycin and clarithromycin, and the ketolide antibiotic telithromycin, HIV protease inhibitors, boceprevir, telaprevir, the antidepressant nefazodone, cobicistat-containing products, or grapefruit juice
[see Clinical Pharmacology (12.3)]. Combination of these drugs with ezetimibe and simvastatin is contraindicated. If short-term treatment with strong CYP3A4 inhibitors is unavoidable, therapy with ezetimibe and simvastatin must be suspended during the course of treatment[see Contraindications (4)andDrug Interactions (7)].The combined use of ezetimibe and simvastatin with gemfibrozil, cyclosporine, or danazol is contraindicated
[see Contraindications (4)andDrug Interactions (7.1and 7.2)].Caution should be used when prescribing fenofibrates with ezetimibe and simvastatin, as these agents can cause myopathy when given alone and the risk is increased when they are co-administered
[see Drug Interactions (7.2, 7.7)].Cases of myopathy, including rhabdomyolysis, have been reported with simvastatin co-administered with colchicine, and caution should be exercised when prescribing ezetimibe and simvastatin with colchicine
[see Drug Interactions (7.9)].The benefits of the combined use of ezetimibe and simvastatin with the following drugs should be carefully weighed against the potential risks of combinations: other lipid-lowering drugs (fenofibrates or, for patients with HoFH, lomitapide), amiodarone, dronedarone, verapamil, diltiazem, amlodipine, or ranolazine
[see Dosage and Administration (2.4), Drug Interactions (7.3)].Cases of myopathy, including rhabdomyolysis, have been observed with simvastatin co-administered with lipid-modifying doses (≥1 g/day niacin) of niacin-containing products
[see Drug Interactions (7.4)].Cases of rhabdomyolysis have been reported with ezetimibe and simvastatin administered with daptomycin. Temporarily suspend ezetimibe and simvastatin in patients taking daptomycin
[see Drug Interactions (7.10)].Prescribing recommendations for interacting agents are summarized in Table 1
[see also Dosage and Administration (2.3, 2.4), Drug Interactions (7)and
,Clinical Pharmacology (12.3)].Table 1: Drug Interactions Associated with Increased Risk of Myopathy/RhabdomyolysisInteracting AgentsPrescribing RecommendationsStrong CYP3A4 Inhibitors, e.g.:
Itraconazole
Ketoconazole
Posaconazole
Voriconazole
Erythromycin
Clarithromycin
Telithromycin
HIV protease inhibitors
Boceprevir
Telaprevir
Nefazodone
Cobicistat-containing products
Gemfibrozil
Cyclosporine
DanazolContraindicated with ezetimibe and simvastatin
Niacin (≥1 g/day)
For Chinese patients, not recommended with ezetimibe and simvastatin
Verapamil
Diltiazem
DronedaroneDo not exceed 10/10 mg ezetimibe and simvastatin daily
Amiodarone
Amlodipine
RanolazineDo not exceed 10/20 mg ezetimibe and simvastatin daily
Lomitapide
For patients with HoFH, do not exceed 10/20 mg ezetimibe and simvastatin daily*
Daptomycin
Temporarily suspend ezetimibe and simvastatin
Grapefruit juice
Avoid grapefruit juice
* For patients with HoFH who have been taking 80 mg simvastatin chronically (e.g., for 12 months or more) without evidence of muscle toxicity, do not exceed 10/40 mg ezetimibe and simvastatin when taking lomitapide.
- Concomitant administration of gemfibrozil, cyclosporine, or danazol [see.]
5.1 Myopathy/RhabdomyolysisSimvastatin occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase above ten times the upper limit of normal (ULN). Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. The risk of myopathy is increased by elevated plasma levels of simvastatin and simvastatin acid. Predisposing factors for myopathy include advanced age (≥65 years), female gender, uncontrolled hypothyroidism, and renal impairment. Chinese patients may be at increased risk for myopathy
[see Use in Specific Populations (8.8)].The risk of myopathy, including rhabdomyolysis, is dose related.In a clinical trial database in which 41,413 patients were treated with simvastatin, 24,747 (approximately 60%) of whom were enrolled in studies with a median follow-up of at least 4 years, the incidence of myopathy was approximately 0.03% and 0.08% at 20 and 40 mg/day, respectively. The incidence of myopathy with 80 mg (0.61%) was disproportionately higher than that observed at the lower doses. In these trials, patients were carefully monitored and some interacting medicinal products were excluded.In a clinical trial in which 12,064 patients with a history of myocardial infarction were treated with simvastatin (mean follow-up 6.7 years), the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum creatine kinase [CK] >10 times upper limit of normal [ULN]) in patients on 80 mg/day was approximately 0.9% compared with 0.02% for patients on 20 mg/day. The incidence of rhabdomyolysis (defined as myopathy with a CK >40 times ULN) in patients on 80 mg/day was approximately 0.4% compared with 0% for patients on 20 mg/day. The incidence of myopathy, including rhabdomyolysis, was highest during the first year and then notably decreased during the subsequent years of treatment. In this trial, patients were carefully monitored and some interacting medicinal products were excluded.
The risk of myopathy, including rhabdomyolysis, is greater in patients on simvastatin 80 mg compared with other statin therapies with similar or greater LDL-C-lowering efficacy and compared with lower doses of simvastatin. Therefore, the 10/80-mg dose of ezetimibe and simvastatinshould be used only in patients who have been taking ezetimibe and simvastatin10/80 mg chronically (e.g., for 12 months or more) without evidence of muscle toxicity[see Dosage and Administration, Restricted Dosing for 10/80 mg ]. If, however, a patient who is currently tolerating the 10/80-mg dose of ezetimibe and simvastatinneeds to be initiated on an interacting drug that is contraindicated or is associated with a dose cap for simvastatin, that patient should be switched to an alternative statin or statin-based regimen with less potential for the drug-drug interaction. Patients should be advised of the increased risk of myopathy, including rhabdomyolysis, and to report promptly any unexplained muscle pain, tenderness or weakness. If symptoms occur, treatment should be discontinued immediately[see Warnings and Precautions (5.2)].In the Study of Heart and Renal Protection (SHARP), 9,270 patients with chronic kidney disease were allocated to receive ezetimibe and simvastatin 10/20 mg daily (n=4,650) or placebo (n=4,620). During a median follow-up period of 4.9 years, the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum creatine kinase [CK] >10 times upper limit of normal [ULN]) was 0.2% for ezetimibe and simvastatin and 0.1% for placebo: the incidence of rhabdomyolysis (defined as myopathy with a CK > 40 times ULN) was 0.09% for ezetimibe and simvastatin and 0.02% for placebo.
In postmarketing experience with ezetimibe, cases of myopathy and rhabdomyolysis have been reported. Most patients who developed rhabdomyolysis were taking a statin prior to initiating ezetimibe. However, rhabdomyolysis has been reported with ezetimibe monotherapy and with the addition of ezetimibe to agents known to be associated with increased risk of rhabdomyolysis, such as fibric acid derivatives. Ezetimibe and simvastatin and a fenofibrate, if taking concomitantly, should both be immediately discontinued if myopathy is diagnosed or suspected.
All patients starting therapy with ezetimibe and simvastatinor whose dose of ezetimibe and simvastatinis being increased should be advised of the risk of myopathy, including rhabdomyolysis, and told to report promptly any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuingezetimibe and simvastatin. Ezetimibe and simvastatintherapy should be discontinued immediately if myopathy is diagnosed or suspected.In most cases, muscle symptoms and CK increases resolved when simvastatin treatment was promptly discontinued. Periodic CK determinations may be considered in patients starting therapy with ezetimibe and simvastatin or whose dose is being increased, but there is no assurance that such monitoring will prevent myopathy.Many of the patients who have developed rhabdomyolysis on therapy with simvastatin have had complicated medical histories, including renal insufficiency usually as a consequence of long-standing diabetes mellitus. Such patients taking ezetimibe and simvastatin merit closer monitoring.
Ezetimibe and simvastatin therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Ezetimibe and simvastatin therapy should also be temporarily withheld in any patient experiencing an acute or serious condition predisposing to the development of renal failure secondary to rhabdomyolysis, e.g., sepsis; hypotension; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy.
Drug InteractionsThe risk of myopathy and rhabdomyolysis is increased by elevated plasma levels of simvastatin and simvastatin acid. Simvastatin is metabolized by the cytochrome P450 isoform 3A4. Certain drugs that inhibit this metabolic pathway can raise the plasma levels of simvastatin and may increase the risk of myopathy. These include itraconazole, ketoconazole, posaconazole, and voriconazole, the macrolide antibiotics erythromycin and clarithromycin, and the ketolide antibiotic telithromycin, HIV protease inhibitors, boceprevir, telaprevir, the antidepressant nefazodone, cobicistat-containing products, or grapefruit juice
[see Clinical Pharmacology (12.3)]. Combination of these drugs with ezetimibe and simvastatin is contraindicated. If short-term treatment with strong CYP3A4 inhibitors is unavoidable, therapy with ezetimibe and simvastatin must be suspended during the course of treatment[see Contraindications (4)andDrug Interactions (7)].The combined use of ezetimibe and simvastatin with gemfibrozil, cyclosporine, or danazol is contraindicated
[see Contraindications (4)andDrug Interactions (7.1and 7.2)].Caution should be used when prescribing fenofibrates with ezetimibe and simvastatin, as these agents can cause myopathy when given alone and the risk is increased when they are co-administered
[see Drug Interactions (7.2, 7.7)].Cases of myopathy, including rhabdomyolysis, have been reported with simvastatin co-administered with colchicine, and caution should be exercised when prescribing ezetimibe and simvastatin with colchicine
[see Drug Interactions (7.9)].The benefits of the combined use of ezetimibe and simvastatin with the following drugs should be carefully weighed against the potential risks of combinations: other lipid-lowering drugs (fenofibrates or, for patients with HoFH, lomitapide), amiodarone, dronedarone, verapamil, diltiazem, amlodipine, or ranolazine
[see Dosage and Administration (2.4), Drug Interactions (7.3)].Cases of myopathy, including rhabdomyolysis, have been observed with simvastatin co-administered with lipid-modifying doses (≥1 g/day niacin) of niacin-containing products
[see Drug Interactions (7.4)].Cases of rhabdomyolysis have been reported with ezetimibe and simvastatin administered with daptomycin. Temporarily suspend ezetimibe and simvastatin in patients taking daptomycin
[see Drug Interactions (7.10)].Prescribing recommendations for interacting agents are summarized in Table 1
[see also Dosage and Administration (2.3, 2.4), Drug Interactions (7)and
,Clinical Pharmacology (12.3)].Table 1: Drug Interactions Associated with Increased Risk of Myopathy/RhabdomyolysisInteracting AgentsPrescribing RecommendationsStrong CYP3A4 Inhibitors, e.g.:
Itraconazole
Ketoconazole
Posaconazole
Voriconazole
Erythromycin
Clarithromycin
Telithromycin
HIV protease inhibitors
Boceprevir
Telaprevir
Nefazodone
Cobicistat-containing products
Gemfibrozil
Cyclosporine
DanazolContraindicated with ezetimibe and simvastatin
Niacin (≥1 g/day)
For Chinese patients, not recommended with ezetimibe and simvastatin
Verapamil
Diltiazem
DronedaroneDo not exceed 10/10 mg ezetimibe and simvastatin daily
Amiodarone
Amlodipine
RanolazineDo not exceed 10/20 mg ezetimibe and simvastatin daily
Lomitapide
For patients with HoFH, do not exceed 10/20 mg ezetimibe and simvastatin daily*
Daptomycin
Temporarily suspend ezetimibe and simvastatin
Grapefruit juice
Avoid grapefruit juice
* For patients with HoFH who have been taking 80 mg simvastatin chronically (e.g., for 12 months or more) without evidence of muscle toxicity, do not exceed 10/40 mg ezetimibe and simvastatin when taking lomitapide.
- Hypersensitivity to any component of this medication [see.]
6.2 Postmarketing ExperienceBecause the below reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following adverse reactions have been reported in postmarketing experience for ezetimibe and simvastatin or ezetimibe or simvastatin: pruritus; alopecia; erythema multiforme; a variety of skin changes (e.g., nodules, discoloration, dryness of skin/mucous membranes, changes to hair/nails); dizziness; muscle cramps; myalgia; arthralgia; pancreatitis; paresthesia; peripheral neuropathy; vomiting; nausea; anemia; erectile dysfunction; interstitial lung disease; myopathy/rhabdomyolysis
[see Warnings and Precautions (5.1)]; hepatitis/jaundice; fatal and non-fatal hepatic failure; depression; cholelithiasis; cholecystitis; thrombocytopenia; elevations in liver transaminases; elevated creatine phosphokinase.There have been rare reports of immune-mediated necrotizing myopathy associated with statin use
[see Warnings and Precautions (5.1)].Hypersensitivity reactions, including anaphylaxis, angioedema, rash, and urticaria have been reported.
In addition, an apparent hypersensitivity syndrome has been reported rarely that has included one or more of the following features: anaphylaxis, angioedema, lupus erythematous-like syndrome, polymyalgia rheumatica, dermatomyositis, vasculitis, purpura, thrombocytopenia, leukopenia, hemolytic anemia, positive ANA, ESR increase, eosinophilia, arthritis, arthralgia, urticaria, asthenia, photosensitivity, fever, chills, flushing, malaise, dyspnea, toxic epidermal necrolysis, erythema multiforme, including Stevens-Johnson syndrome.
There have been rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).
- Active liver disease or unexplained persistent elevations in hepatic transaminase levels [see.]
5.3 Liver EnzymesIn three placebo-controlled, 12-week trials, the incidence of consecutive elevations (≥3 X ULN) in serum transaminases was 1.7% overall for patients treated with ezetimibe and simvastatin and appeared to be dose-related with an incidence of 2.6% for patients treated with ezetimibe and simvastatin 10/80. In controlled long-term (48-week) extensions, which included both newly-treated and previously-treated patients, the incidence of consecutive elevations (≥3 X ULN) in serum transaminases was 1.8% overall and 3.6% for patients treated with ezetimibe and simvastatin 10/80. These elevations in transaminases were generally asymptomatic, not associated with cholestasis, and returned to baseline after discontinuation of therapy or with continued treatment.
In SHARP, 9,270 patients with chronic kidney disease were allocated to receive ezetimibe and simvastatin 10/20 mg daily (n=4,650), or placebo (n=4,620). During a median follow-up period of 4.9 years, the incidence of consecutive elevations of transaminases (>3 X ULN) was 0.7% for ezetimibe and simvastatin and 0.6% for placebo.
It is recommended that liver function tests be performed before the initiation of treatment with ezetimibe and simvastatin, and thereafter when clinically indicated. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including simvastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with ezetimibe and simvastatin, promptly interrupt therapy. If an alternate etiology is not found do not restart ezetimibe and simvastatin. Note that ALT may emanate from muscle, therefore ALT rising with CK may indicate myopathy
[see Warnings and Precautions (5.1)].Ezetimibe and simvastatin should be used with caution in patients who consume substantial quantities of alcohol and/or have a past history of liver disease. Active liver diseases or unexplained persistent transaminase elevations are contraindications to the use of ezetimibe and simvastatin.
- Women who are pregnant or may become pregnant. Serum cholesterol and triglycerides increase during normal pregnancy, and cholesterol or cholesterol derivatives are essential for fetal development. Because HMG-CoA reductase inhibitors (statins), such as simvastatin, decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, ezetimibe and simvastatin may cause fetal harm when administered to a pregnant woman. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hypercholesterolemia. There are no adequate and well-controlled studies of ezetimibe and simvastatin use during pregnancy; however, in rare reports congenital anomalies were observed following intrauterine exposure to statins. In rat and rabbit animal reproduction studies, simvastatin revealed no evidence of teratogenicity. Ezetimibe and simvastatinshould be administered to women of childbearing age only when such patients are highly unlikely to conceive.If the patient becomes pregnant while taking this drug, ezetimibe and simvastatin should be discontinued immediately and the patient should be apprised of the potential hazard to the fetus[see Use in Specific Populations (8.1)].
- Nursing mothers. It is not known whether simvastatin is excreted into human milk; however, a small amount of another drug in this class does pass into breast milk. Because statins have the potential for serious adverse reactions in nursing infants, women who require ezetimibe and simvastatin treatment should not breastfeed their infants [see Use in Specific Populations (8.3)].
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Rhabdomyolysis and myopathy [see]
5.1 Myopathy/RhabdomyolysisSimvastatin occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase above ten times the upper limit of normal (ULN). Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. The risk of myopathy is increased by elevated plasma levels of simvastatin and simvastatin acid. Predisposing factors for myopathy include advanced age (≥65 years), female gender, uncontrolled hypothyroidism, and renal impairment. Chinese patients may be at increased risk for myopathy
[see Use in Specific Populations (8.8)].The risk of myopathy, including rhabdomyolysis, is dose related.In a clinical trial database in which 41,413 patients were treated with simvastatin, 24,747 (approximately 60%) of whom were enrolled in studies with a median follow-up of at least 4 years, the incidence of myopathy was approximately 0.03% and 0.08% at 20 and 40 mg/day, respectively. The incidence of myopathy with 80 mg (0.61%) was disproportionately higher than that observed at the lower doses. In these trials, patients were carefully monitored and some interacting medicinal products were excluded.In a clinical trial in which 12,064 patients with a history of myocardial infarction were treated with simvastatin (mean follow-up 6.7 years), the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum creatine kinase [CK] >10 times upper limit of normal [ULN]) in patients on 80 mg/day was approximately 0.9% compared with 0.02% for patients on 20 mg/day. The incidence of rhabdomyolysis (defined as myopathy with a CK >40 times ULN) in patients on 80 mg/day was approximately 0.4% compared with 0% for patients on 20 mg/day. The incidence of myopathy, including rhabdomyolysis, was highest during the first year and then notably decreased during the subsequent years of treatment. In this trial, patients were carefully monitored and some interacting medicinal products were excluded.
The risk of myopathy, including rhabdomyolysis, is greater in patients on simvastatin 80 mg compared with other statin therapies with similar or greater LDL-C-lowering efficacy and compared with lower doses of simvastatin. Therefore, the 10/80-mg dose of ezetimibe and simvastatinshould be used only in patients who have been taking ezetimibe and simvastatin10/80 mg chronically (e.g., for 12 months or more) without evidence of muscle toxicity[see Dosage and Administration, Restricted Dosing for 10/80 mg ]. If, however, a patient who is currently tolerating the 10/80-mg dose of ezetimibe and simvastatinneeds to be initiated on an interacting drug that is contraindicated or is associated with a dose cap for simvastatin, that patient should be switched to an alternative statin or statin-based regimen with less potential for the drug-drug interaction. Patients should be advised of the increased risk of myopathy, including rhabdomyolysis, and to report promptly any unexplained muscle pain, tenderness or weakness. If symptoms occur, treatment should be discontinued immediately[see Warnings and Precautions (5.2)].In the Study of Heart and Renal Protection (SHARP), 9,270 patients with chronic kidney disease were allocated to receive ezetimibe and simvastatin 10/20 mg daily (n=4,650) or placebo (n=4,620). During a median follow-up period of 4.9 years, the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum creatine kinase [CK] >10 times upper limit of normal [ULN]) was 0.2% for ezetimibe and simvastatin and 0.1% for placebo: the incidence of rhabdomyolysis (defined as myopathy with a CK > 40 times ULN) was 0.09% for ezetimibe and simvastatin and 0.02% for placebo.
In postmarketing experience with ezetimibe, cases of myopathy and rhabdomyolysis have been reported. Most patients who developed rhabdomyolysis were taking a statin prior to initiating ezetimibe. However, rhabdomyolysis has been reported with ezetimibe monotherapy and with the addition of ezetimibe to agents known to be associated with increased risk of rhabdomyolysis, such as fibric acid derivatives. Ezetimibe and simvastatin and a fenofibrate, if taking concomitantly, should both be immediately discontinued if myopathy is diagnosed or suspected.
All patients starting therapy with ezetimibe and simvastatinor whose dose of ezetimibe and simvastatinis being increased should be advised of the risk of myopathy, including rhabdomyolysis, and told to report promptly any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuingezetimibe and simvastatin. Ezetimibe and simvastatintherapy should be discontinued immediately if myopathy is diagnosed or suspected.In most cases, muscle symptoms and CK increases resolved when simvastatin treatment was promptly discontinued. Periodic CK determinations may be considered in patients starting therapy with ezetimibe and simvastatin or whose dose is being increased, but there is no assurance that such monitoring will prevent myopathy.Many of the patients who have developed rhabdomyolysis on therapy with simvastatin have had complicated medical histories, including renal insufficiency usually as a consequence of long-standing diabetes mellitus. Such patients taking ezetimibe and simvastatin merit closer monitoring.
Ezetimibe and simvastatin therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Ezetimibe and simvastatin therapy should also be temporarily withheld in any patient experiencing an acute or serious condition predisposing to the development of renal failure secondary to rhabdomyolysis, e.g., sepsis; hypotension; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy.
Drug InteractionsThe risk of myopathy and rhabdomyolysis is increased by elevated plasma levels of simvastatin and simvastatin acid. Simvastatin is metabolized by the cytochrome P450 isoform 3A4. Certain drugs that inhibit this metabolic pathway can raise the plasma levels of simvastatin and may increase the risk of myopathy. These include itraconazole, ketoconazole, posaconazole, and voriconazole, the macrolide antibiotics erythromycin and clarithromycin, and the ketolide antibiotic telithromycin, HIV protease inhibitors, boceprevir, telaprevir, the antidepressant nefazodone, cobicistat-containing products, or grapefruit juice
[see Clinical Pharmacology (12.3)]. Combination of these drugs with ezetimibe and simvastatin is contraindicated. If short-term treatment with strong CYP3A4 inhibitors is unavoidable, therapy with ezetimibe and simvastatin must be suspended during the course of treatment[see Contraindications (4)andDrug Interactions (7)].The combined use of ezetimibe and simvastatin with gemfibrozil, cyclosporine, or danazol is contraindicated
[see Contraindications (4)andDrug Interactions (7.1and 7.2)].Caution should be used when prescribing fenofibrates with ezetimibe and simvastatin, as these agents can cause myopathy when given alone and the risk is increased when they are co-administered
[see Drug Interactions (7.2, 7.7)].Cases of myopathy, including rhabdomyolysis, have been reported with simvastatin co-administered with colchicine, and caution should be exercised when prescribing ezetimibe and simvastatin with colchicine
[see Drug Interactions (7.9)].The benefits of the combined use of ezetimibe and simvastatin with the following drugs should be carefully weighed against the potential risks of combinations: other lipid-lowering drugs (fenofibrates or, for patients with HoFH, lomitapide), amiodarone, dronedarone, verapamil, diltiazem, amlodipine, or ranolazine
[see Dosage and Administration (2.4), Drug Interactions (7.3)].Cases of myopathy, including rhabdomyolysis, have been observed with simvastatin co-administered with lipid-modifying doses (≥1 g/day niacin) of niacin-containing products
[see Drug Interactions (7.4)].Cases of rhabdomyolysis have been reported with ezetimibe and simvastatin administered with daptomycin. Temporarily suspend ezetimibe and simvastatin in patients taking daptomycin
[see Drug Interactions (7.10)].Prescribing recommendations for interacting agents are summarized in Table 1
[see also Dosage and Administration (2.3, 2.4), Drug Interactions (7)and
,Clinical Pharmacology (12.3)].Table 1: Drug Interactions Associated with Increased Risk of Myopathy/RhabdomyolysisInteracting AgentsPrescribing RecommendationsStrong CYP3A4 Inhibitors, e.g.:
Itraconazole
Ketoconazole
Posaconazole
Voriconazole
Erythromycin
Clarithromycin
Telithromycin
HIV protease inhibitors
Boceprevir
Telaprevir
Nefazodone
Cobicistat-containing products
Gemfibrozil
Cyclosporine
DanazolContraindicated with ezetimibe and simvastatin
Niacin (≥1 g/day)
For Chinese patients, not recommended with ezetimibe and simvastatin
Verapamil
Diltiazem
DronedaroneDo not exceed 10/10 mg ezetimibe and simvastatin daily
Amiodarone
Amlodipine
RanolazineDo not exceed 10/20 mg ezetimibe and simvastatin daily
Lomitapide
For patients with HoFH, do not exceed 10/20 mg ezetimibe and simvastatin daily*
Daptomycin
Temporarily suspend ezetimibe and simvastatin
Grapefruit juice
Avoid grapefruit juice
* For patients with HoFH who have been taking 80 mg simvastatin chronically (e.g., for 12 months or more) without evidence of muscle toxicity, do not exceed 10/40 mg ezetimibe and simvastatin when taking lomitapide.
- Liver enzyme abnormalities [see]
5.3 Liver EnzymesIn three placebo-controlled, 12-week trials, the incidence of consecutive elevations (≥3 X ULN) in serum transaminases was 1.7% overall for patients treated with ezetimibe and simvastatin and appeared to be dose-related with an incidence of 2.6% for patients treated with ezetimibe and simvastatin 10/80. In controlled long-term (48-week) extensions, which included both newly-treated and previously-treated patients, the incidence of consecutive elevations (≥3 X ULN) in serum transaminases was 1.8% overall and 3.6% for patients treated with ezetimibe and simvastatin 10/80. These elevations in transaminases were generally asymptomatic, not associated with cholestasis, and returned to baseline after discontinuation of therapy or with continued treatment.
In SHARP, 9,270 patients with chronic kidney disease were allocated to receive ezetimibe and simvastatin 10/20 mg daily (n=4,650), or placebo (n=4,620). During a median follow-up period of 4.9 years, the incidence of consecutive elevations of transaminases (>3 X ULN) was 0.7% for ezetimibe and simvastatin and 0.6% for placebo.
It is recommended that liver function tests be performed before the initiation of treatment with ezetimibe and simvastatin, and thereafter when clinically indicated. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including simvastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with ezetimibe and simvastatin, promptly interrupt therapy. If an alternate etiology is not found do not restart ezetimibe and simvastatin. Note that ALT may emanate from muscle, therefore ALT rising with CK may indicate myopathy
[see Warnings and Precautions (5.1)].Ezetimibe and simvastatin should be used with caution in patients who consume substantial quantities of alcohol and/or have a past history of liver disease. Active liver diseases or unexplained persistent transaminase elevations are contraindications to the use of ezetimibe and simvastatin.
Ezetimibe and simvastatin tablets contains ezetimibe USP, a selective inhibitor of intestinal cholesterol and related phytosterol absorption, and simvastatin USP, an HMG-CoA reductase inhibitor.
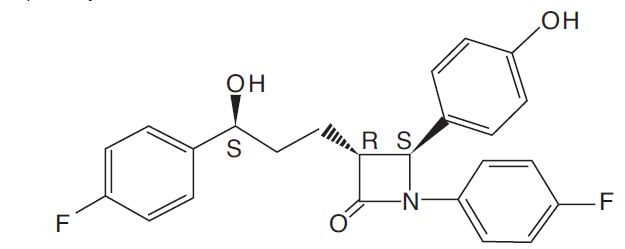
The chemical name of ezetimibe is 1-(4-fluorophenyl)-3(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone. The molecular formula is C
24H
21F
2NO
3 and its molecular weight is 409.4.
Ezetimibe, USP is a white, crystalline powder that is freely to very soluble in ethanol, methanol, and acetone and practically insoluble in water. Its structural formula is:
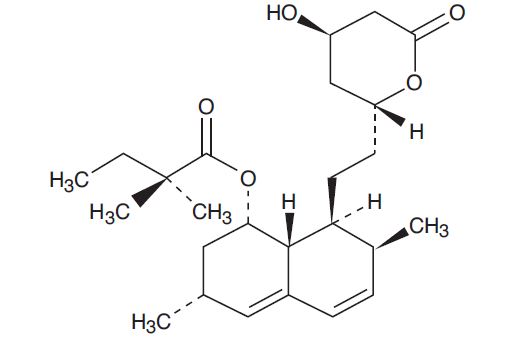
Simvastatin, USP an inactive lactone, is hydrolyzed to the corresponding β-hydroxyacid form, which is an inhibitor of HMG-CoA reductase. Simvastatin, USP is butanoic acid, 2,2-dimethyl-,1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2
25H
38O
5 and its molecular weight is 418.57.
Simvastatin, USP is a white to off-white, nonhygroscopic, crystalline powder that is practically insoluble in water and freely soluble in chloroform, methanol and ethanol. Its structural formula is:
Ezetimibe and simvastatin is available for oral use as tablets containing 10 mg of ezetimibe, USP, and 10 mg of simvastatin, USP (ezetimibe and simvastatin 10/10), 20 mg of simvastatin, USP (ezetimibe and simvastatin 10/20), 40 mg of simvastatin, USP (ezetimibe and simvastatin 10/40), or 80 mg of simvastatin, USP (ezetimibe and simvastatin 10/80). Each tablet contains the following inactive ingredients: butylated hydroxyanisole, citric acid monohydrate, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and propyl gallate.