Felbamate Prescribing Information
THE USE OF FELBAMATE IS ASSOCIATED WITH A MARKED INCREASE IN THE INCIDENCE OF APLASTIC ANEMIA. ACCORDINGLY, FELBAMATE SHOULD ONLY BE USED IN PATIENTS WHOSE EPILEPSY IS SO SEVERE THAT THE RISK OF APLASTIC ANEMIA IS DEEMED ACCEPTABLE IN LIGHT OF THE BENEFITS CONFERRED BY ITS USE (SEE
AMONG FELBAMATE TREATED PATIENTS, APLASTIC ANEMIA (PANCYTOPENIA IN THE PRESENCE OF A BONE MARROW LARGELY DEPLETED OF HEMATOPOIETIC PRECURSORS) OCCURS AT AN INCIDENCE THAT MAY BE MORE THAN A 100 FOLD GREATER THAN THAT SEEN IN THE UNTREATED POPULATION (I.E., 2 TO 5 PER MILLION PERSONS PER YEAR). THE RISK OF DEATH IN PATIENTS WITH APLASTIC ANEMIA GENERALLY VARIES AS A FUNCTION OF ITS SEVERITY AND ETIOLOGY; CURRENT ESTIMATES OF THE OVERALL CASE FATALITY RATE ARE IN THE RANGE OF 20 TO 30%, BUT RATES AS HIGH AS 70% HAVE BEEN REPORTED IN THE PAST.
THERE ARE TOO FEW FELBAMATE ASSOCIATED CASES, AND TOO LITTLE KNOWN ABOUT THEM TO PROVIDE A RELIABLE ESTIMATE OF THE SYNDROME'S INCIDENCE OR ITS CASE FATALITY RATE OR TO IDENTIFY THE FACTORS, IF ANY, THAT MIGHT CONCEIVABLY BE USED TO PREDICT WHO IS AT GREATER OR LESSER RISK.
IN MANAGING PATIENTS ON FELBAMATE, IT SHOULD BE BORNE IN MIND THAT THE CLINICAL MANIFESTATION OF APLASTIC ANEMIA MAY NOT BE SEEN UNTIL AFTER A PATIENT HAS BEEN ON FELBAMATE FOR SEVERAL MONTHS (E.G., ONSET OF APLASTIC ANEMIA AMONG FELBAMATE EXPOSED PATIENTS FOR WHOM DATA ARE AVAILABLE HAS RANGED FROM 5 TO 30 WEEKS). HOWEVER, THE INJURY TO BONE MARROW STEM CELLS THAT IS HELD TO BE ULTIMATELY RESPONSIBLE FOR THE ANEMIA MAY OCCUR WEEKS TO MONTHS EARLIER. ACCORDINGLY, PATIENTS WHO ARE DISCONTINUED FROM FELBAMATE REMAIN AT RISK FOR DEVELOPING ANEMIA FOR A VARIABLE, AND UNKNOWN, PERIOD AFTERWARDS.
IT IS NOT KNOWN WHETHER OR NOT THE RISK OF DEVELOPING APLASTIC ANEMIA CHANGES WITH DURATION OF EXPOSURE. CONSEQUENTLY, IT IS NOT SAFE TO ASSUME THAT A PATIENT WHO HAS BEEN ON FELBAMATE WITHOUT SIGNS OF HEMATOLOGIC ABNORMALITY FOR LONG PERIODS OF TIME IS WITHOUT RISK.
IT IS NOT KNOWN WHETHER OR NOT THE DOSE OF FELBAMATE AFFECTS THE INCIDENCE OF APLASTIC ANEMIA.
IT IS NOT KNOWN WHETHER OR NOT CONCOMITANT USE OF ANTIEPILEPTIC DRUGS AND/OR OTHER DRUGS AFFECTS THE INCIDENCE OF APLASTIC ANEMIA.
APLASTIC ANEMIA TYPICALLY DEVELOPS WITHOUT PREMONITORY CLINICAL OR LABORATORY SIGNS, THE FULL BLOWN SYNDROME PRESENTING WITH SIGNS OF INFECTION, BLEEDING, OR ANEMIA. ACCORDINGLY, ROUTINE BLOOD TESTING CANNOT BE RELIABLY USED TO REDUCE THE INCIDENCE OF APLASTIC ANEMIA, BUT, IT WILL, IN SOME CASES, ALLOW THE DETECTION OF THE HEMATOLOGIC CHANGES BEFORE THE SYNDROME DECLARES ITSELF CLINICALLY. FELBAMATE SHOULD BE DISCONTINUED IF ANY EVIDENCE OF BONE MARROW DEPRESSION OCCURS.
EVALUATION OF POSTMARKETING EXPERIENCE SUGGESTS THAT ACUTE LIVER FAILURE IS ASSOCIATED WITH THE USE OF FELBAMATE. THE REPORTED RATE IN THE U.S. HAS BEEN ABOUT 6 CASES OF LIVER FAILURE LEADING TO DEATH OR TRANSPLANT PER 75,000 PATIENT YEARS OF USE. THIS RATE IS AN UNDERESTIMATE BECAUSE OF UNDER REPORTING, AND THE TRUE RATE COULD BE CONSIDERABLY GREATER THAN THIS. FOR EXAMPLE, IF THE REPORTING RATE IS 10%, THE TRUE RATE WOULD BE ONE CASE PER 1,250 PATIENT YEARS OF USE.
OF THE CASES REPORTED, ABOUT 67% RESULTED IN DEATH OR LIVER TRANSPLANTATION, USUALLY WITHIN 5 WEEKS OF THE ONSET OF SIGNS AND SYMPTOMS OF LIVER FAILURE. THE EARLIEST ONSET OF SEVERE HEPATIC DYSFUNCTION FOLLOWED SUBSEQUENTLY BY LIVER FAILURE WAS 3 WEEKS AFTER INITIATION OF FELBAMATE. ALTHOUGH SOME REPORTS DESCRIBED DARK URINE AND NONSPECIFIC PRODROMAL SYMPTOMS (E.G., ANOREXIA, MALAISE, AND GASTROINTESTINAL SYMPTOMS), IN OTHER REPORTS IT WAS NOT CLEAR IF ANY PRODROMAL SYMPTOMS PRECEDED THE ONSET OF JAUNDICE.
IT IS NOT KNOWN WHETHER OR NOT THE RISK OF DEVELOPING HEPATIC FAILURE CHANGES WITH DURATION OF EXPOSURE.
IT IS NOT KNOWN WHETHER OR NOT THE DOSAGE OF FELBAMATE AFFECTS THE INCIDENCE OF HEPATIC FAILURE.
IT IS NOT KNOWN WHETHER CONCOMITANT USE OF OTHER ANTIEPILEPTIC DRUGS AND/OR OTHER DRUGS AFFECT THE INCIDENCE OF HEPATIC FAILURE.
FELBAMATE SHOULD NOT BE PRESCRIBED FOR ANYONE WITH A HISTORY OF HEPATIC DYSFUNCTION.
TREATMENT WITH FELBAMATE SHOULD BE INITIATED ONLY IN INDIVIDUALS WITHOUT ACTIVE LIVER DISEASE AND WITH NORMAL BASELINE SERUM TRANSAMINASES. IT HAS NOT BEEN PROVED THAT PERIODIC SERUM TRANSAMINASE TESTING WILL PREVENT SERIOUS INJURY BUT IT IS GENERALLY BELIEVED THAT EARLY DETECTION OF DRUGINDUCED HEPATIC INJURY ALONG WITH IMMEDIATE WITHDRAWAL OF THE SUSPECT DRUG ENHANCES THE LIKELIHOOD FOR RECOVERY. THERE IS NO INFORMATION AVAILABLE THAT DOCUMENTS HOW RAPIDLY PATIENTS CAN PROGRESS FROM NORMAL LIVER FUNCTION TO LIVER FAILURE, BUT OTHER DRUGS KNOWN TO BE HEPATOTOXINS CAN CAUSE LIVER FAILURE RAPIDLY (E.G., FROM NORMAL ENZYMES TO LIVER FAILURE IN 2-4 WEEKS). ACCORDINGLY, MONITORING OF SERUM TRANSAMINASE LEVELS (AST AND ALT) IS RECOMMENDED AT BASELINE AND PERIODICALLY THEREAFTER. WHILE THE MORE FREQUENT THE MONITORING THE GREATER THE CHANCES OF EARLY DETECTION, THE PRECISE SCHEDULE FOR MONITORING IS A MATTER OF CLINICAL JUDGEMENT.
FELBAMATE SHOULD BE DISCONTINUED IF EITHER SERUM AST OR SERUM ALT LEVELS BECOME INCREASED ≥ 2 TIMES THE UPPER LIMIT OF NORMAL, OR IF CLINICAL SIGNS AND SYMPTOMS SUGGEST LIVER FAILURE (SEE PRECAUTIONS). PATIENTS WHO DEVELOP EVIDENCE OF HEPATOCELLULAR INJURY WHILE ON FELBAMATE AND ARE WITHDRAWN FROM THE DRUG FOR ANY REASON SHOULD BE PRESUMED TO BE AT INCREASED RISK FOR LIVER INJURY IF FELBAMATE IS REINTRODUCED. ACCORDINGLY, SUCH PATIENTS SHOULD NOT BE CONSIDERED FOR RE-TREATMENT.
Felbamate oral suspension is not indicated as a first line antiepileptic treatment (see
If these criteria are met and the patient has been fully advised of the risk, and has provided written acknowledgement, felbamate oral suspension can be considered for either monotherapy or adjunctive therapy in the treatment of partial seizures, with and without generalization, in adults with epilepsy and as adjunctive therapy in the treatment of partial and generalized seizures associated with Lennox-Gastaut syndrome in children.
Felbamate oral suspension has been studied as monotherapy and adjunctive therapy in adults and as adjunctive therapy in children with seizures associated with Lennox-Gastaut syndrome. As felbamate oral suspension is added to or substituted for existing AEDs, it is strongly recommended to reduce the dosage of those AEDs in the range of 20-33% to minimize side effects (see
The majority of patients received 3600 mg/day in clinical trials evaluating its use as both monotherapy and adjunctive therapy.
Table 6 Dosage Table (adults) | |||
| Dosage reduction of concomitant AEDs | WEEK 1 REDUCE original dose by 20–33%* | WEEK 2 REDUCE original dose by up to an additional 1/3* | WEEK 3 REDUCE as clinically indicated |
| Felbamate Dosage | 1200 mg/dayInitial dose | 2400 mg/day Therapeutic dosage range | 3600 mg/day Therapeutic dosage range |
| *See Adjunctive Conversion to Monotherapy | |||
While the above felbamate conversion guidelines may result in a felbamate 3600 mg/day dose within 3 weeks, in some patients titration to a 3600 mg/day felbamate dose has been achieved in as little as 3 days with appropriate adjustment of other AEDs.
Felbamate oral suspension is contraindicated in patients with known hypersensitivity to felbamate, its ingredients, or known sensitivity to other carbamates. It should not be used in patients with a history of any blood dyscrasia or hepatic dysfunction.
The most common adverse reactions seen in association with felbamate in adults during monotherapy are anorexia, vomiting, insomnia, nausea, and headache. The most common adverse reactions seen in association with felbamate in adults during adjunctive therapy are anorexia, vomiting, insomnia, nausea, dizziness, somnolence, and headache.
The most common adverse reactions seen in association with felbamate in children during adjunctive therapy are anorexia, vomiting, insomnia, headache, and somnolence.
The dropout rate because of adverse experiences or intercurrent illnesses among adult felbamate patients was 12 percent (120/977). The dropout rate because of adverse experiences or intercurrent illnesses among pediatric felbamate patients was six percent (22/357). In adults, the body systems associated with causing these withdrawals in order of frequency were: digestive (4.3%), psychological (2.2%), whole body (1.7%), neurological (1.5%), and dermatological (1.5%). In children, the body systems associated with causing these withdrawals in order of frequency were: digestive (1.7%), neurological (1.4%), dermatological (1.4%), psychological (1.1%), and whole body (1.0%). In adults, specific events with an incidence of 1% or greater associated with causing these withdrawals, in order of frequency were: anorexia (1.6%), nausea (1.4%), rash (1.2%), and weight decrease (1.1%). In children, specific events with an incidence of 1% or greater associated with causing these withdrawals, in order of frequency was rash (1.1%).
The prescriber should be aware that the figures cited in the following table cannot be used to predict the incidence of side effects in the course of usual medical practice where patient characteristics and other factors differ from those which prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different investigators, treatments, and uses including the use of felbamate as adjunctive therapy where the incidence of adverse events may be higher due to drug interactions. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and nondrug factors to the side effect incidence rate in the population studied.
The table that follows enumerates adverse events that occurred at an incidence of 2% or more among 58 adult patients who received felbamate monotherapy at dosages of 3600 mg/day in double-blind controlled trials. Table 3 presents reported adverse events that were classified using standard WHO-based dictionary terminology.
Table 3 Adults Treatment-Emergent Adverse Event Incidence in Controlled Monotherapy Trials | ||
| | Felbamate* (N=58) | Low Dose Valproate** (N=50) |
| Body System Event | % | % |
| Body as a Whole | | |
| Fatigue | 6.9 | 4.0 |
| Weight Decrease | 3.4 | 0 |
| Face Edema | 3.4 | 0 |
| Central Nervous System | | |
| Insomnia | 8.6 | 4.0 |
| Headache | 6.9 | 18.0 |
| Anxiety | 5.2 | 2.0 |
| Dermatological | | |
| Acne | 3.4 | 0 |
| Rash | 3.4 | 0 |
| Digestive | | |
| Dyspepsia | 8.6 | 2.0 |
| Vomiting | 8.6 | 2.0 |
| Constipation | 6.9 | 2.0 |
| Diarrhea | 5.2 | 0 |
| SGPT Increased | 5.2 | 2.0 |
| Metabolic/Nutritional | | |
| Hypophosphatemia | 3.4 | 0 |
| Respiratory | | |
| Upper Respiratory Tract Infection | 8.6 | 4.0 |
| Rhinitis | 6.9 | 0 |
| Special Senses | | |
| Diplopia | 3.4 | 4.0 |
| Otitis Media | 3.4 | 0 |
| Urogenital | | |
| Intramenstrual Bleeding | 3.4 | 0 |
| Urinary Tract Infection | 3.4 | 2.0 |
| *3600 mg/day;** 15 mg/kg/day | ||
Table 4 enumerates adverse events that occurred at an incidence of 2% or more among 114 adult patients who received felbamate adjunctive therapy in add-on controlled trials at dosages up to 3600 mg/day. Reported adverse events were classified using standard WHO-based dictionary terminology.
Many adverse experiences that occurred during adjunctive therapy may be a result of drug interactions. Adverse experiences during adjunctive therapy typically resolved with conversion to monotherapy, or with adjustment of the dosage of other antiepileptic drugs.
Table 4 Adults Treatment-Emergent Adverse Event Incidence in Controlled Add-On Trials | ||
| | Felbamate | Placebo |
| | (N=114) | (N=43) |
| Body System/Event | % | % |
| Body as a Whole | | |
| Fatigue | 16.8 | 7.0 |
| Fever | 2.6 | 4.7 |
| Chest Pain | 2.6 | 0 |
| Central Nervous System | | |
| Headache | 36.8 | 9.3 |
| Somnolence | 19.3 | 7.0 |
| Dizziness | 18.4 | 14.0 |
| Insomnia | 17.5 | 7.0 |
| Nervousness | 7.0 | 2.3 |
| Tremor | 6.1 | 2.3 |
| Anxiety | 5.3 | 4.7 |
| Gait Abnormal | 5.3 | 0 |
| Depression | 5.3 | 0 |
| Paraesthesia | 3.5 | 2.3 |
| Ataxia | 3.5 | 0 |
| Mouth Dry | 2.6 | 0 |
| Stupor | 2.6 | 0 |
| Dermatological | | |
| Rash | 3.5 | 4.7 |
| Digestive | | |
| Nausea | 34.2 | 2.3 |
| Anorexia | 19.3 | 2.3 |
| Vomiting | 16.7 | 4.7 |
| Dyspepsia | 12.3 | 7.0 |
| Constipation | 11.4 | 2.3 |
| Diarrhea | 5.3 | 2.3 |
| Abdominal Pain | 5.3 | 0 |
| SGPT Increased | 3.5 | 0 |
| Musculoskeletal | | |
| Myalgia | 2.6 | 0 |
| Respiratory | | |
| Upper Respiratory Tract Infection | 5.3 | 7.0 |
| Sinusitis | 3.5 | 0 |
| Pharyngitis | 2.6 | 0 |
| Special Senses | | |
| Diplopia | 6.1 | 0 |
| Taste Perversion | 6.1 | 0 |
| Vision Abnormal | 5.3 | 2.3 |
Table 5 enumerates adverse events that occurred more than once among 31 pediatric patients who received felbamate up to 45 mg/kg/day or a maximum of 3600 mg/day. Reported adverse events were classified using standard WHO-based dictionary terminology.
Table 5 Children Treatment-Emergent Adverse Event Incidence in Controlled Add-On Lennox-Gastaut Trials | ||
| | Felbamate | Placebo |
| | (N=31) | (N=27) |
| Body System/Event | % | % |
| Body as a Whole Fever Fatigue Weight Decrease Pain | 22.6 9.7 6.5 6.5 | 11.1 3.7 0 0 |
| Central Nervous System | | |
| Somnolence | 48.4 | 11.1 |
| Insomnia | 16.1 | 14.8 |
| Nervousness | 16.1 | 18.5 |
| Gait Abnormal | 9.7 | 0 |
| Headache | 6.5 | 18.5 |
| Thinking Abnormal | 6.5 | 3.7 |
| Ataxia | 6.5 | 3.7 |
| Urinary Incontinence | 6.5 | 7.4 |
| Emotional Lability | 6.5 | 0 |
| Miosis | 6.5 | 0 |
| Dermatological | | |
| Rash | 9.7 | 7.4 |
| Digestive | | |
| Anorexia | 54.8 | 14.8 |
| Vomiting | 38.7 | 14.8 |
| Constipation | 12.9 | 0 |
| Hiccup | 9.7 | 3.7 |
| Nausea | 6.5 | 0 |
| Dyspepsia | 6.5 | 3.7 |
| Hematologic | | |
| Purpura | 12.9 | 7.4 |
| Leukopenia | 6.5 | 0 |
| Respiratory | | |
| Upper Respiratory Tract Infection | 45.2 | 25.9 |
| Pharyngitis | 9.7 | 3.7 |
| Coughing | 6.5 | 0 |
| Special Senses | | |
| Otitis Media | 9.7 | 0 |
In the paragraphs that follow, the adverse clinical events, other than those in the preceding tables, that occurred in a total of 977 adults and 357 children exposed to felbamate and that are reasonably associated with its use are presented. They are listed in order of decreasing frequency. Because the reports cite events observed in open-label and uncontrolled studies, the role of felbamate in their causation cannot be reliably determined.
Events are classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring on one or more occasions in at least 1/100 patients; infrequent adverse events are those occurring in 1/100-1/1000 patients; and rare events are those occurring in fewer than 1/1000 patients.
Event frequencies are calculated as the number of patients reporting an event divided by the total number of patients (N=1334) exposed to felbamate.
Voluntary reports of adverse events in patients taking felbamate (usually in conjunction with other drugs) have been received since market introduction and may have no causal relationship with the drug(s). These include the following by body system:
epidermal necrolysis.
hyperglycemia, hypocalcemia.
Felbamate, USP is an antiepileptic available as a 600 mg/5 mL suspension for oral administration. Its chemical name is 2-phenyl-1,3-propanediol dicarbamate.
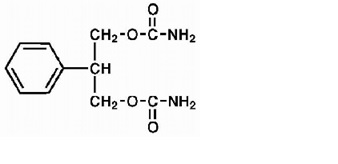
Felbamate, USP is a white to off-white crystalline powder with a characteristic odor. It is very slightly soluble in water, slightly soluble in ethanol, sparingly soluble in methanol, and freely soluble in dimethyl sulfoxide. The molecular weight is 238.24; felbamate, USP’s molecular formula is C11H14N2O4; its structural formula is:
The inactive ingredients for Felbamate Oral Suspension, USP 600 mg/5 mL are noncrystallizing sorbitol solution, microcrystalline cellulose and carboxymethylcellulose sodium, glycerin, methylparaben, propylparaben, polysorbate 80, simethicone emulsion, saccharin sodium monohydrate, bubblegum flavor (contains arabic gum, and natural and artificial flavor), FD&C Red No. 40, FD&C Yellow No. 6, and purified water.