Fenofibrate
Fenofibrate Prescribing Information
Fenofibrate capsules are indicated as adjunctive therapy to diet for the reduction of LDL-C, Total-C, Triglycerides and Apo B in adult patients with primary hypercholesterolemia or mixed dyslipidemia (Fredrickson Types IIa and IIb). Lipid-altering agents should be used in addition to a diet restricted in saturated fat and cholesterol when response to diet and non-pharmacological interventions alone has been inadequate (see National Cholesterol Education Program [NCEP] Treatment Guidelines, below).
Fenofibrate capsules are also indicated as adjunctive therapy to diet for treatment of adult patients with hypertriglyceridemia (Fredrickson Types IV and V hyperlipidemia).
Improving glycemic control in diabetic patients showing fasting chylomicronemia will usually reduce fasting triglycerides and eliminate chylomicronemia thereby obviating the need for pharmacologic intervention.
Markedly elevated levels of serum triglycerides (e.g. > 2,000 mg/dL) may increase the risk of developing pancreatitis. The effect of fenofibrate therapy on reducing this risk has not been adequately studied.
Drug therapy is not indicated for patients with Type I hyperlipoproteinemia, who have elevations of chylomicrons and plasma triglycerides, but who have normal levels of very low density lipoprotein (VLDL). Inspection of plasma refrigerated for 14 hours is helpful in distinguishing Types I, IV and V hyperlipoproteinemia2.
The initial treatment for dyslipidemia is dietary therapy specific for the type of lipoprotein abnormality. Excess body weight and excess alcoholic intake may be important factors in hypertriglyceridemia and should be addressed prior to any drug therapy. Physical exercise can be an important ancillary measure. Diseases contributory to hyperlipidemia, such as hypothyroidism or diabetes mellitus should be looked for and adequately treated. Estrogen therapy, like thiazide diuretics and beta-blockers, is sometimes associated with massive rises in plasma triglycerides, especially in subjects with familial hypertriglyceridemia. In such cases, discontinuation of the specific etiologic agent may obviate the need for specific drug therapy of hypertriglyceridemia.
The use of drugs should be considered only when reasonable attempts have been made to obtain satisfactory results with non-drug methods. If the decision is made to use drugs, the patient should be instructed that this does not reduce the importance of adhering to diet (see WARNINGS and PRECAUTIONS).
Fredrickson Classification of Hyperlipoproteinemias
Lipid Elevation | |||
Type | Lipoprotein Elevated | Major | Minor |
| I (rare) | chylomicrons | TG | ↑↔C |
| IIa | LDL | C | — |
| IIb | LDL, VLDL | C | TG |
| III (rare) | IDL | C, TG | — |
| IV | VLDL | TG | ↑↔C |
| V (rare) | Chylomicrons, VLDL | TG | ↑↔ |
C = cholesterol
TG = triglycerides
LDL = low density lipoprotein
VLDL = very low density lipoprotein
IDL = intermediate density lipoprotein
| Definite Athlerosclerotic Diseasea | Two or More Other Risk Factors b | LDL-Cholesterol mg/dL (mmol/L) | |
Initiation Level | Goal | ||
| No | No | ≥ 190 (≥ 4.9) | < 160 (< 4.1) |
| No | Yes | ≥ 160 (≥ 4.1) | < 130 (< 3.4) |
| Yes | Yes or No | ≥ 130c (≥ 3.4) | < 100 (< 2.6) |
a Coronary heart disease or peripheral vascular disease (including symptomatic carotid artery disease).
b Other risk factors for coronary heart disease (CHD) include: age (males: ≥ 45 years; females: ≥ 55 years or premature menopause without estrogen replacement therapy); family history of premature CHD; current cigarette smoking; hypertension; confirmed HDL-C <35 mg/dL (<0.91mmol/L); and diabetes mellitus. Subtract 1 risk factor if HDL-C is ≥ 60 mg/dL (≥1.6 mmol/L)
c In CHD patients with LDL-C levels 100 to 129 mg/dL, the physician should exercise clinical judgment in deciding whether to initiate drug treatment.
Patients should be placed on an appropriate lipid-lowering diet before receiving fenofibrate capsules, and should continue this diet during treatment with fenofibrate capsules. Fenofibrate capsules should be given with meals, thereby optimizing the bioavailability of the medication.
For the treatment of adult patients with primary hypercholesterolemia or mixed hyperlipidemia, the initial dose of fenofibrate capsules is 200 mg per day.
For adult patients with hypertriglyceridemia, the initial dose is 67 to 200 mg per day. Dosage should be individualized according to patient response, and should be adjusted if necessary following repeat lipid determinations at 4 to 8 week intervals. The maximum dose is 200 mg per day.
Treatment with fenofibrate capsules should be initiated at a dose of 67 mg/day in patients having impaired renal function, and increased only after evaluation of the effects on renal function and lipid levels at this dose. In the elderly, the initial dose should likewise be limited to 67 mg/day.
Lipid levels should be monitored periodically and consideration should be given to reducing the dosage of fenofibrate capsules if lipid levels fall significantly below the targeted range.
Fenofibrate capsules are contraindicated in patients who exhibit hypersensitivity to fenofibrate.
Fenofibrate capsules are contraindicated in patients with hepatic or severe renal dysfunction, including primary biliary cirrhosis, and patients with unexplained persistent liver function abnormality.
Fenofibrate capsules are contraindicated in patients with preexisting gallbladder disease (see WARNINGS).
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Adverse events reported by 2% or more of patients treated with fenofibrate (and greater than placebo) during the double-blind, placebo-controlled trials, regardless of causality, are listed in Table 3 below. Adverse events led to discontinuation of treatment in 5% of patients treated with fenofibrate and in 3% treated with placebo. Increases in liver function tests were the most frequent events, causing discontinuation of fenofibrate treatment in 1.6% of patients in double-blind trials.
Photosensitivity reactions have occurred days to months after initiation; in some of these cases,
patients reported a prior photosensitivity reaction to ketoprofen.BODY SYSTEM Adverse Reaction | Fenofibrate* (N=439) | PLACEBO (N=365) |
BODY AS A WHOLE | | |
| Abdominal Pain | 4.6% | 4.4% |
| Back Pain | 3.4% | 2.5% |
| Headache | 3.2% | 2.7% |
DIGESTIVE | | |
| Abnormal Liver Function Tests | 7.5% ** | 1.4% |
| Nausea | 2.3% | 1.9% |
| Constipation | 2.1% | 1.4% |
METABOLIC AND NUTRITIONAL DISORDERS | ||
| Increased ALT | 3% | 1.6% |
| Increased CPK | 3% | 1.4% |
| Increased AST | 3.4% ** | 0.5% |
RESPIRATORY | | |
| Respiratory Disorder | 6.2% | 5.5% |
| Rhinitis | 2.3% | 1.1% |
* Dosage equivalent to 145 mg fenofibrate
** Significantly different from Placebo
Increases in Liver Enzymes
In a pooled analysis of 10 placebo-controlled trials, increases to >3 times the upper limit of normal in ALT occurred in 5.3% of patients taking fenofibrate versus 1.1% of patients treated with placebo. In an 8-week study, the incidence of ALT or AST elevations ≥ 3 times the upper limit of normal was 13% in patients receiving dosages equivalent to 134 mg to 200 mg fenofibrate daily and was 0% in those receiving dosages equivalent to 34 mg to 67 mg fenofibrate daily or placebo.
The following adverse reactions have been identified during post-approval use of fenofibrate: myalgia, rhabdomyolysis, pancreatitis, acute renal failure, muscle spasm, hepatitis, cirrhosis, increased total bilirubin, anemia, arthralgia, decreases in hemoglobin, decreases in hematocrit, white blood cell decreases, asthenia and interstitial lung disease. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
To report SUSPECTED ADVERSE REACTIONS, contact Alembic Pharmaceuticals Limited at 1-866-210-9797 or FDA at 1-800-FDA-1088 or
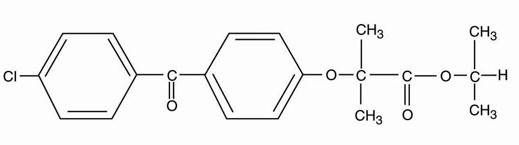
Fenofibrate, USP (micronized), is a lipid regulating agent available as capsules for oral administration. Each capsule contains 67 mg, 134 mg or 200 mg of micronized fenofibrate, USP. The chemical name for fenofibrate, USP is 2-[4-(4-chlorobenzoyl) phenoxy]-2-methyl-propanoic acid,1-methylethyl ester with the following structural formula:
The empirical formula is C20H21O4Cl and the molecular weight is 360.83; fenofibrate, USP is very soluble in methylene chloride; slightly soluble in alcohol; practically insoluble in water. The melting point is 79 to 82°C. Fenofibrate, USP is a white or almost white crystalline powder which is stable under ordinary conditions.
Each 67 mg fenofibrate capsule, USP (micronized) contains the following inactive ingredients: microcrystalline cellulose, croscarmellose sodium, hypromellose, sodium lauryl sulphate, magnesium stearate, titanium dioxide, gelatin, FD&C Yellow No. 6 and D&C Yellow No.10.
Each 134 mg fenofibrate capsule, USP (micronized) contains the following inactive ingredients: microcrystalline cellulose, croscarmellose sodium, hypromellose, sodium lauryl sulphate, magnesium stearate, titanium dioxide and gelatin.
Each 200 mg fenofibrate capsule, USP (micronized) contains the following inactive ingredients: microcrystalline cellulose, croscarmellose sodium, hypromellose, sodium lauryl sulphate, magnesium stearate, D&C Red No. 28, FD&C Red No. 40, D&C Yellow No.10, titanium dioxide and gelatin.
The printing ink contains shellac, potassium hydroxide and iron oxide black.
Fenofibrate capsules, USP meets USP
A variety of clinical studies have demonstrated that elevated levels of total cholesterol (total- C), low density lipoprotein cholesterol (LDL-C), and apolipoprotein B (apo B), an LDL membrane complex, are associated with human atherosclerosis. Similarly, decreased levels of high density lipoprotein cholesterol (HDL-C) and its transport complex, apolipoprotein A (apo AI and apo AII) are associated with the development of atherosclerosis. Epidemiologic investigations have established that cardiovascular morbidity and mortality vary directly with the level of total-C, LDL-C, and triglycerides, and inversely with the level of HDL-C. The independent effect of raising HDL-C or lowering triglycerides (TG) on the risk of cardiovascular morbidity and mortality has not been determined.
Fenofibric acid, the active metabolite of fenofibrate, produces reductions in total cholesterol, LDL cholesterol, apolipoprotein B, total triglycerides and triglyceride rich lipoprotein (VLDL) in treated patients. In addition, treatment with fenofibrate results in increases in high density lipoprotein (HDL) and apoproteins apo AI and apo AII).
The effects of fenofibric acid seen in clinical practice have been explained
Fenofibrate also reduces serum uric acid levels in hyperuricemic and normal individuals by increasing the urinary excretion of uric acid.
Clinical experience has been obtained with two different formulations of fenofibrate: a “micronized” and “non-micronized” formulation, which have been demonstrated to be bioequivalent. Comparisons of blood levels following oral administration of both formulations in healthy volunteers demonstrate that a single capsule containing 67 mg of the “micronized” formulation is bioequivalent to 100 mg of the “non-micronized” formulation. Three capsules containing 67 mg fenofibrate (micronized) are bioequivalent to a single 200 mg fenofibrate (micronized) capsule.
The absolute bioavailability of fenofibrate cannot be determined as the compound is virtually insoluble in aqueous media suitable for injection. However, fenofibrate is well absorbed from the gastrointestinal tract. Following oral administration in healthy volunteers, approximately 60% of a single dose of radiolabelled fenofibrate appeared in urine, primarily as fenofibric acid and its glucuronate conjugate, and 25% was excreted in the feces. Peak plasma levels of fenofibric acid occur within 6 to 8 hours after administration.
The absorption of fenofibrate is increased when administered with food. With micronized fenofibrate, the absorption is increased by approximately 35% under fed as compared to fasting conditions.
In healthy volunteers, steady-state plasma levels of fenofibric acid were shown to be achieved within 5 days of dosing with single oral doses equivalent to 67 mg of fenofibrate and did not demonstrate accumulation across time following multiple dose administration. Serum protein binding was approximately 99% in normal and hyperlipidemic subjects.
Following oral administration, fenofibrate is rapidly hydrolyzed by esterases to the active metabolite, fenofibric acid; no unchanged fenofibrate is detected in plasma.
Fenofibric acid is primarily conjugated with glucuronic acid and then excreted in urine. A small amount of fenofibric acid is reduced at the carbonyl moiety to a benzhydrol metabolite which is, in turn, conjugated with glucuronic acid and excreted in urine.
After absorption, fenofibrate is mainly excreted in the urine in the form of metabolites, primarily fenofibric acid and fenofibric acid glucuronide. After administration of radiolabelled fenofibrate, approximately 60% of the dose appeared in the urine and 25% was excreted in the feces.
Fenofibric acid is eliminated with a half-life of 20 hours, allowing once daily administration in a clinical setting.
In elderly volunteers 77 to 87 years of age, the oral clearance of fenofibric acid following a single oral dose of fenofibrate was 1.2 L/h, which compares to 1.1 L/h in young adults. This indicates that a similar dosage regimen can be used in the elderly, without increasing accumulation of the drug or metabolites.
Fenofibrate has not been investigated in adequate and well-controlled trials in pediatric patients.
No pharmacokinetic difference between males and females has been observed for fenofibrate.
The influence of race on the pharmacokinetics of fenofibrate has not been studied, however fenofibrate is not metabolized by enzymes known for exhibiting inter-ethnic variability. Therefore, inter-ethnic pharmacokinetic differences are very unlikely.
The pharmacokinetics of fenofibric acid was examined in patients with mild, moderate and severe renal impairment. Patients with severe renal impairment (creatinine clearance [CrCl] ≤ 30 mL/min) showed 2.7-fold increase in exposure for fenofibric acid and increased accumulation of fenofibric acid during chronic dosing compared to that of healthy subjects. Patients with mild to moderate renal impairment (CrCl 30 to 80 ml/min) had similar exposure but an increase in the half-life for fenofibric acid compared to that of healthy subjects. Based on these findings, the use of fenofibrate should be avoided in patients who have severe renal impairment and dose reduction is required in patients having mild to moderate renal impairment.
No pharmacokinetic studies have been conducted in patients having hepatic insufficiency.
Potentiation of coumarin-type anti-coagulants has been observed with prolongation of the prothrombin time/INR.
Bile acid sequestrants have been shown to bind other drugs given concurrently. Therefore, fenofibrate should be taken at least 1 hour before or 4 to 6 hours after a bile acid binding resin to avoid impeding its absorption (see WARNINGS and PRECAUTIONS).
Concomitant administration of a single dose of fenofibrate (administered as 3 x 67 mg fenofibrate capsules) with a single dose of pravastatin (40 mg) in 23 healthy subjects increased the mean Cmax and mean AUC for pravastatin by 13%. The Cmax and AUC of fenofibrate decreased by 2% and 1%, respectively, after concomitant administration of fenofibrate and pravastatin. The mean Cmax and AUC for 3α-hydroxy-iso-pravastatin increased by 29% and 26%, respectively.
Concomitant administration of a single dose of fenofibrate (equivalent to 145 mg fenofibrate) and a single dose of fluvastatin (40 mg) resulted in a small increase (approximately 15% to 16%) in exposure to (+)3R,5S-fluvastatin, the active enantiomer of fluvastatin.
A single dose of either pravastatin or fluvastatin had no clinically important effect on the pharmacokinetics of fenofibric acid.
Concomitant administration of fenofibrate (equivalent to fenofibrate 200 mg) with atorvastatin (20 mg) once daily for 10 days resulted in approximately 17% decrease (range from 67% decrease to 44% increase) in atorvastatin AUC values in 22 healthy males. The atorvastatin Cmax values were not significantly affected by fenofibrate. The pharmacokinetics of fenofibric acid were not significantly affected by atorvastatin.
Concomitant administration of fenofibrate (equivalent to fenofibrate 200 mg) once daily for 10 days with glimepiride (1 mg tablet) single dose simultaneously with the last dose of fenofibrate resulted in a 35% increase in mean AUC of glimepiride in healthy subjects. Glimepiride Cmax was not significantly affected by fenofibrate coadministration. There was no statistically significant effect of multiple doses of fenofibrate on glucose nadir or AUC with the baseline glucose concentration as the covariate after glimepiride administration in healthy volunteers. However, glucose concentrations at 24 hours remained statistically significantly lower after pretreatment with fenofibrate than with glimepiride alone. Glimepiride had no significant effect on the pharmacokinetics of fenofibric acid.
Concomitant administration of fenofibrate (54 mg) and metformin (850 mg) 3 times a day for 10 days resulted in no significant changes in the pharmacokinetics of fenofibric acid and metformin when compared with the two drugs administered alone in healthy subjects.
Concomitant administration of fenofibrate (equivalent to fenofibrate 200 mg) once daily for 14 days with rosiglitazone tablet (rosiglitazone maleate) (8 mg) once daily for 5 days, Day 10 through Day 14, resulted in no significant changes in the pharmacokinetics of fenofibric acid and rosiglitazone when compared with the two drugs administered alone in healthy subjects.
The effects of fenofibrate at a dose equivalent to 200 mg fenofibrate capsules per day were assessed from four randomized, placebo-controlled, double-blind, parallel-group studies including patients with the following mean baseline lipid values: total-C 306.9 mg/dL; LDL-C 213.8 mg/dL; HDL-C 52.3 mg/dL; and triglycerides 191 mg/dL. Fenofibrate capsules therapy lowered LDL-C, Total-C and the LDL-C/HDL-C ratio. Fenofibrate capsules therapy also lowered triglycerides and raised HDL-C (see Table 1).
Treatment Group | Total-C | LDL-C | HDL-C | TG |
|---|---|---|---|---|
| Pooled Cohort | | |||
| Mean baseline lipid values (n=646) | 306.9 mg/dL | 213.8 mg/dL | 52.3 mg/dL | 191 mg/dL |
| All FEN (n=361) | -18.7%* | -20.6%* | +11%* | -28.9%* |
| Placebo (n=285) | -0.4% | -2.2% | +0.7% | +7.7% |
| Baseline LDL-C >160 mg/dL and TG <150 mg/dL (Type IIa) | ||||
| Mean baseline lipid values (n=334) | 307.7 mg/dL | 227.7 mg/dL | 58.1 mg/dL | 101.7 mg/dL |
| All FEN (n=193) | -22.4%* | -31.4%* | +9.8% | -23.5%* |
| Placebo (n=141) | +0.2% | -2.2% | +2.6% | +11.7% |
| Baseline LDL-C > 160 mg/dL and TG ≥ 150 mg/dL (Type IIb) | ||||
| Mean baseline lipid values (n=646) | 312.8 mg/dL | 219.8 mg/dL | 46.7 mg/dL | 231.9 mg/dL |
| All FEN (n=126) | -16.8%* | -20.1%* | +14.6%* | -35.9%* |
| Placebo (n=116) | -3% | -6.6% | +2.3% | +0.9% |
*p = <0.05 vs. Placebo
In a subset of the subjects, measurements of apo B were conducted. Fenofibrate treatment significantly reduced apo B from baseline to endpoint as compared with placebo (-25.1% vs. 2.4%, p<0.0001, n=213 and 143 respectively).
The effects of fenofibrate on serum triglycerides were studied in two randomized, double-blind, placebo-controlled clinical trials1 of 147 hypertriglyceridemia patients (Fredrickson Types IV and V). Patients were treated for eight weeks under protocols that differed only in that one entered patients with baseline triglyceride (TG) levels of 500 to 1500 mg/dL, and the other TG levels of 350 to 500 mg/dL. In patients with hypertriglyceridemia and normal cholesterolemia with or without hyperchylomicronemia (Type IV/V hyperlipidemia), treatment with fenofibrate at dosages equivalent to 200 mg fenofibrate per day decreased primarily very low density lipoprotein (VLDL) triglycerides and VLDL cholesterol. Treatment of patients with Type IV hyperlipoproteinemia and elevated triglycerides often results in an increase of low density lipoprotein (LDL) cholesterol (see Table 2).
Study 1 Baseline TG levels 350 to 499 mg/dL | Placebo | Fenofibrate Capsules | |||||||
N | Baseline (Mean) | Endpoint (Mean) | % Change (Mean) | N | Baseline (Mean) | Endpoint (Mean) | % Change (Mean) | ||
| Triglycerides | 28 | 449 | 450 | -0.5 | 27 | 432 | 223 | -46.2* | |
| VLDL Triglycerides | 19 | 367 | 350 | 2.7 | 19 | 350 | 178 | -44.1* | |
| Total Cholesterol | 28 | 255 | 261 | 2.8 | 27 | 252 | 227 | -9.1* | |
| HDL Cholesterol | 28 | 35 | 36 | 4 | 27 | 34 | 40 | 19.6* | |
| LDL Cholesterol | 28 | 120 | 129 | 12 | 27 | 128 | 137 | 14.5 | |
| VLDL Cholesterol | 27 | 99 | 99 | 5.8 | 27 | 92 | 46 | -44.7* | |
Study 2 Baseline TG levels 500 to 1500 mg/dL | Placebo | Fenofibrate Capsules | |||||||
N | Baseline (Mean) | Endpoint (Mean) | % Change (Mean) | N | Baseline (Mean) | Endpoint (Mean) | % Change (Mean) | ||
| Triglycerides | 44 | 710 | 750 | 7.2 | 48 | 726 | 308 | -54.5* | |
| VLDL Triglycerides | 29 | 537 | 571 | 18.7 | 33 | 543 | 205 | -50.6* | |
| Total Cholesterol | 44 | 272 | 271 | 0.4 | 48 | 261 | 223 | -13.8* | |
| HDL Cholesterol | 44 | 27 | 28 | 5 | 48 | 30 | 36 | 22.9* | |
| LDL Cholesterol | 42 | 100 | 90 | -4.2 | 45 | 103 | 131 | 45* | |
| VLDL Cholesterol | 42 | 137 | 142 | 11 | 45 | 126 | 54 | -49.4* | |
* = p <0.05 vs. Placebo
The effect of fenofibrate on cardiovascular morbidity and mortality has not been determined.