Fensolvi
(Leuprolide Acetate)Fensolvi Prescribing Information
FENSOLVI is indicated for the treatment of pediatric patients 2 years of age and older with central precocious puberty (CPP).
For injectable suspension, 45 mg of leuprolide acetate is supplied in a kit containing:
- Syringe A contains diluent for reconstitution (ATRIGEL Delivery System) in a prefilled syringe.
- Syringe B contains 45 mg lyophilized leuprolide acetate powder in a single-dose prefilled syringe.
- Hypersensitivity to GnRH, GnRH agonists or any of the components of FENSOLVI. Anaphylactic reactions to synthetic GnRH or GnRH agonists have been reported [see Adverse Reactions (.)]
6.2 Postmarketing ExperienceThe following adverse reactions have been identifed during postapproval use of leuprolide acetate or FENSOLVI. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Allergic Reactions:anaphylactic, rash, urticaria, and photosensitivity reactions.General:chest pain, weight increase, weight decrease, decreased appetite, fatigue.Laboratory Abnormalities:decreased WBC.Metabolic: diabetes mellitus.Musculoskeletal and Connective Tissue:arthralgia, epiphysiolysis, muscle spasms, myalgia.Neurologic:neuropathy peripheral, convulsion, paralysis, insomnia, pseudotumor cerebri (idiopathic intracranial hypertension).Psychiatric:emotional lability, such as crying, irritability, impatience, anger and aggression. Depression, including rare reports of suicidal ideation and attempt. Many, but not all, of these patients had a history of psychiatric illness or other comorbidities with an increased risk of depression.Skin and Subcutaneous Tissue:injection site reactions including induration and abscess, flushing, hyperhidrosis.Reproductive System:vaginal bleeding, breast enlargement.Vascular:hypertension, hypotension.Respiratory:dyspnea. - Pregnancy: FENSOLVI may cause fetal harm[see Use in Specific Populations (.)]
8.1 PregnancyRisk SummaryFENSOLVI is contraindicated in pregnancy
[see Contraindications ].FENSOLVI may cause fetal harm based on findings from animal studies and the drug’s mechanism of action
[see Clinical Pharmacology ].The available data from published clinical studies and case reports and from the pharmacovigilance database on exposure to leuprolide acetate during pregnancy are insufficient to assess the risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Based on animal reproduction studies, leuprolide acetate may be associated with an increased risk of pregnancy complications, including early pregnancy loss and fetal harm. In animal reproduction studies, subcutaneous administration of leuprolide acetate to rabbits during the period of organogenesis caused embryo-fetal toxicity, decreased fetal weights and a dose-dependent increase in major fetal abnormalities in animals at doses less than the recommended human dose based on body surface area using an estimated daily dose. A similar rat study also showed increased fetal mortality and decreased fetal weights but no major fetal abnormalities at doses less than the recommended human dose based on body surface area using an estimated daily dose(see Data).The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
DataAnimal DataWhen administered on day 6 of pregnancy at test dosages of 0.00024 mg/kg, 0.0024 mg/kg, and 0.024 mg/kg (1/3255 to 1/33 of the human dose) to rabbits, leuprolide acetate produced a dose-related increase in major fetal abnormalities. Similar studies in rats failed to demonstrate an increase in fetal malformations. There was increased fetal mortality and decreased fetal weights with the two higher doses of leuprolide acetate in rabbits and with the highest dose (0.024 mg/kg) in rats.
The following serious adverse reactions are described here and elsewhere in the label:
- Initial rise in gonadotropin and sex steroid levels [see Warnings and Precautions (.)]
5.1 Initial Rise of Gonadotropins and Sex Steroid LevelsDuring the early phase of therapy, gonadotropins and sex steroids rise above baseline because of the initial stimulatory effect of the drug
[see Clinical Pharmacology]. Therefore, an increase in clinical signs and symptoms of puberty including vaginal bleeding may be observed during the first weeks of therapy or after subsequent doses[see Adverse Reactions(6)]. Instruct patients and caregivers to notify the physician if these symptoms continue beyond the second month after FENSOLVI administration. - Psychiatric Events [see Warnings and Precautions (.)]
5.2 Psychiatric EventsPsychiatric events have been reported in patients taking GnRH agonists, including leuprolide acetate. Postmarketing reports with this class of drugs include symptoms of emotional lability, such as crying, irritability, impatience, anger, and aggression. Monitor for development or worsening of psychiatric symptoms during treatment with FENSOLVI
[see Adverse Reactions(6.1, 6.2)]. - Convulsions [see Warnings and Precautions (.)]
5.3 ConvulsionsPostmarketing reports of convulsions have been observed in patients receiving GnRH agonists, including leuprolide acetate. These included patients with a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs. Convulsions have also been reported in patients in the absence of any of the conditions mentioned above
[see Adverse Reactions(6.2)]. - Pseudotumor Cerebri (Idiopathic Intracranial Hypertension) [see Warnings and Precautions ()]
5.4 Pseudotumor Cerebri
(Idiopathic Intracranial Hypertension)Pseudotumor cerebri (idiopathic intracranial hypertension) have been reported in pediatric patients receiving GnRH agonists, including leuprolide acetate. Monitor patients for signs and symptoms of pseudotumor cerebri, including headache, papilledema, blurred vision, diplopia, loss of vision, pain behind the eye or pain with eye movement, tinnitus, dizziness, and nausea.
No pharmacokinetic drug-drug interaction studies have been conducted with FENSOLVI.
FENSOLVI for injectable suspension is a sterile polymeric matrix formulation of leuprolide acetate, a GnRH agonist, for subcutaneous use. It is designed to deliver leuprolide acetate at a controlled rate over a six-month therapeutic period.
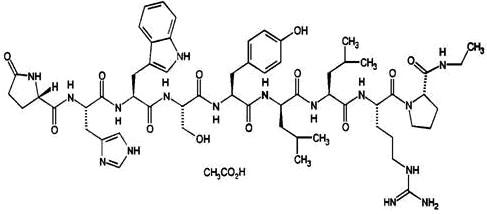
Leuprolide acetate is a synthetic nonapeptide analog of naturally occurring gonadotropin releasing hormone. The analog possesses greater potency than the natural hormone. The chemical name is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) with the following structural formula:
FENSOLVI is prefilled and supplied in two separate, sterile syringes whose contents are mixed immediately prior to administration. The two syringes are joined and the single dose product is mixed until it is homogenous. FENSOLVI is administered subcutaneously, where it forms a solid drug delivery depot.
One syringe contains the ATRIGEL Delivery System and the other contains the leuprolide acetate. ATRIGEL is a polymeric (non-gelatin containing) delivery system consisting of a biodegradable poly(DL-lactide-co-glycolide) (PLG) polymer formulation dissolved in the biocompatible solvent, N-methyl-2-pyrrolidone (NMP).
Refer to Table 3 for the delivery system composition and reconstituted product formulation for FENSOLVI product.
ATRIGEL Delivery System Syringe | Polymer | PLG |
| Polymer description | Copolymer with hexanediol | |
| Polymer DL-lactide to glycolide molar ratio | 85:15 | |
Reconstituted Product | Polymer delivered | 165 mg |
| NMP delivered | 165 mg | |
| Leuprolide acetate delivered | 45 mg | |
| Approximate leuprolide free base equivalent | 42 mg | |
| Approximate administered formulation weight | 375 mg | |
| Approximate injection volume | 0.375 mL |