Fluconazole
Fluconazole Prescribing Information
Fluconazole for oral suspension is indicated for the treatment of:
- Oropharyngeal and esophageal candidiasis. In open noncomparative studies of relatively small numbers of patients, fluconazole for oral suspension was also effective for the treatment of Candidaurinary tract infections, peritonitis, and systemicCandidainfections including candidemia, disseminated candidiasis, and pneumonia.
- Cryptococcal meningitis. Before prescribing fluconazole for oral suspension for AIDS patients withcryptococcal meningitis, please seesection. Studies comparing fluconazole for oral suspension to amphotericin B in non-HIV infected patients have not been conducted.
CLINICAL STUDIESCryptococcal meningitis:In a multicenter study comparing fluconazole (200 mg/day) to amphotericin B (0.3 mg/kg/day) for treatment ofcryptococcal meningitisin patients with AIDS, a multivariate analysis revealed three pretreatment factors that predicted death during the course of therapy: abnormal mental status, cerebrospinal fluid cryptococcal antigen titer greater than 1:1024, and cerebrospinal fluid white blood cell count of less than 20 cells/mm3. Mortality among high risk patients was 33% and 40% for amphotericin B and fluconazole patients, respectively (p=0.58), with overall deaths 14% (9 of 63 subjects) and 18% (24 of 131 subjects) for the 2 arms of the study (p=0.48). Optimal doses and regimens for patients with acutecryptococcal meningitisand at high risk for treatment failure remain to be determined. (Saag,et al. N Engl J Med 1992; 326:83-9.)Pediatric StudiesOropharyngeal candidiasis:An open-label, comparative study of the efficacy and safety of fluconazole (2 to 3 mg/kg/day) and oral nystatin (400,000 I.U. 4 times daily) was conducted in immunocompromised pediatric patients from 6 months to 13 years of age with oropharyngeal candidiasis. Clinical and mycological response rates were higher in pediatric patients treated with fluconazole.
Clinical cure at the end of treatment was reported for 86% of fluconazole-treated patients compared to 46% of nystatin treated patients. Mycologically, 76% of fluconazole treated patients had the infecting organism eradicated compared to 11% for nystatin treated patients.FluconazoleNystatin* Subjects without follow-up cultures for any reason were considered nonevaluable for mycological response. Enrolled 96 90 Clinical Cure 76/88 (86%) 36/78 (46%) Mycological eradication* 55/72 (76%) 6/54 (11%) The proportion of patients with clinical relapse 2 weeks after the end of treatment was 14% for subjects receiving fluconazole and 16% for subjects receiving nystatin. At 4 weeks after the end of treatment, the percentages of patients with clinical relapse were 22% for fluconazole and 23% for nystatin.
Prophylaxis: Fluconazole for oral suspension is also indicated to decrease the incidence of candidiasis in patients undergoing bone marrow transplantation who receive cytotoxic chemotherapy and/or radiation therapy.
Specimens for fungal culture and other relevant laboratory studies (serology, histopathology) should be obtained prior to therapy to isolate and identify causative organisms. Therapy may be instituted before the results of the cultures and other laboratory studies are known; however, once these results become available, anti-infective therapy should be adjusted accordingly.
SINCE ORAL ABSORPTION IS RAPID AND ALMOST COMPLETE, THE DAILY DOSE OF FLUCONAZOLE IS THE SAME FOR ORAL (TABLETS AND SUSPENSION) AND INTRAVENOUS ADMINISTRATION. In general, a loading dose of twice the daily dose is recommended on the first day of therapy to result in plasma concentrations close to steady-state by the second day of therapy.
The daily dose of fluconazole for oral suspension for the treatment of infections should be based on the infecting organism and the patient’s response to therapy. Treatment should be continued until clinical parameters or laboratory tests indicate that active fungal infection has subsided. An inadequate period of treatment may lead to recurrence of active infection. Patients with AIDS and
Oropharyngeal candidiasis
Esophageal candidiasis
Systemic Candida infections
Urinary tract infections and peritonitis
Cryptococcal meningitis
Prophylaxis in patients undergoing bone marrow transplantation
Fluconazole for oral suspension is contraindicated in patients who have shown hypersensitivity to fluconazole or to any of its excipients. There is no information regarding cross-hypersensitivity between fluconazole and other azole antifungal agents. Caution should be used in prescribing fluconazole for oral suspension to patients with hypersensitivity to other azoles. Coadministration of other drugs known to prolong the QT interval and which are metabolized via the enzyme CYP3A4 such as erythromycin, pimozide, and quinidine are contraindicated in patients receiving fluconazole. (See
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Some azoles, including fluconazole, have been associated with prolongation of the QT interval on the electrocardiogram. Fluconazole causes QT prolongation via the inhibition of Rectifier Potassium Channel current (Ikr). The QT prolongation caused by other medicinal products (such as amiodarone) may be amplified via the inhibition of cytochrome P450 (CYP) 3A4. (See
Fluconazole should be administered with caution to patients with these potentially proarrhythmic conditions.
Concomitant use of fluconazole and erythromycin has the potential to increase the risk of cardiotoxicity (prolonged QT interval, torsade de pointes) and consequently sudden heart death. This combination should be avoided.
Fluconazole should be administered with caution to patients with renal dysfunction.
Adrenal insufficiency has been reported in patients receiving azoles, including fluconazole. Reversible cases of adrenal insufficiency have been reported in patients receiving fluconazole.
Fluconazole for Oral Suspension contains sucrose and should not be used in patients with hereditary fructose, glucose/galactose malabsorption, and sucrase-isomaltase deficiency.
When driving vehicles or operating machines, it should be taken into account that occasionally dizziness or seizures may occur.
There have been reports of cases of superinfection with
Abrocitinib
Alfentanil
Azithromycin
Coumarin-type anticoagulants:
HMG-CoA reductase inhibitors
Hydrochlorothiazide
Ibrutinib:
Ivacaftor and fixed dose ivacaftor combinations (e.g.,tezacaftor/ivacaftor and ivacaftor/tezacaftor/elexacaftor):
Lemborexant:
Losartan
Lurasidone:
Methadone
Although not specifically studied, fluconazole has the potential to increase the systemic exposure of other non-steroidal anti-inflammatory drugs (NSAIDs) that are metabolized by CYP2C9 (e.g., naproxen, lornoxicam, meloxicam, diclofenac). Frequent monitoring for adverse events and toxicity related to NSAIDs is recommended. Adjustment of dosage of NSAIDs may be needed.
Theophylline:
Triazolam:
Physicians should be aware that interaction studies with medications other than those listed in the
Fluconazole showed no evidence of carcinogenic potential in mice and rats treated orally for 24 months at doses of 2.5 mg/kg/day, 5 mg/kg/day, or 10 mg/kg/day (approximately 2 to 7 times the recommended human dose). Male rats treated with 5 mg/kg/day and 10 mg/kg/day had an increased incidence of hepatocellular adenomas.
Fluconazole, with or without metabolic activation, was negative in tests for mutagenicity in four strains of
Fluconazole did not affect the fertility of male or female rats treated orally with daily doses of 5 mg/kg, 10 mg/kg, or 20 mg/kg or with parenteral doses of 5 mg/kg, 25 mg/kg, or 75 mg/kg, although the onset of parturition was slightly delayed at 20 mg/kg PO. In an intravenous perinatal study in rats at 5 mg/kg, 20 mg/kg, and 40 mg/kg, dystocia and prolongation of parturition were observed in a few dams at 20 mg/kg (approximately 5 to 15 times the recommended human dose) and 40 mg/kg, but not at 5 mg/kg. The disturbances in parturition were reflected by a slight increase in the number of still born pups and decrease of neonatal survival at these dose levels. The effects on parturition in rats are consistent with the species specific estrogen-lowering property produced by high doses of fluconazole. Such a hormone change has not been observed in women treated with fluconazole. (See
Potential for Fetal Harm: Use in pregnancy should be avoided except in patients with severe or potentially life-threatening fungal infections in whom fluconazole may be used if the anticipated benefit outweighs the possible risk to the fetus. A few published case reports describe a pattern of distinct congenital anomalies in infants exposed
Human Data
Case reports describe a distinctive and rare pattern of birth defects among infants whose mothers received high-dose (400 to 800 mg/day) fluconazole during most or all of the first trimester of pregnancy. The features seen in these infants include brachycephaly, abnormal facies, abnormal calvarial development, cleft palate, femoral bowing, thin ribs and long bones, arthrogryposis, and congenital heart disease. These effects are similar to those seen in animal studies.
Epidemiological studies suggest a potential risk of spontaneous abortion and congenital abnormalities in infants whose mothers were treated with 150 mg of fluconazole as a single or repeated dose in the first trimester, but these epidemiological studies have limitations and these findings have not been confirmed in controlled clinical trials.
Animal Data
Fluconazole was administered orally to pregnant rabbits during organogenesis in two studies at doses of 5 mg/kg, 10 mg/kg, and 20 mg/kg and at 5 mg/kg, 25 mg/kg, and 75 mg/kg, respectively. Maternal weight gain was impaired at all dose levels (approximately 0.25 to 4 times the 400 mg clinical dose based on body surface area [BSA] comparison), and abortions occurred at 75 mg/kg (approximately 4 times the 400 mg clinical dose based on BSA); no adverse fetal effects were observed.
In several studies in which pregnant rats received fluconazole orally during organogenesis, maternal weight gain was impaired and placental weights were increased at 25 mg/kg. There were no fetal effects at 5 mg/kg or 10 mg/kg; increases in fetal anatomical variants (supernumerary ribs, renal pelvis dilation) and delays in ossification were observed at 25 mg/kg and 50 mg/kg and higher doses. At doses ranging from 80 to 320 mg/kg (approximately 2 to 8 times the 400 mg clinical dose based on BSA), embryolethality in rats was increased and fetal abnormalities included wavy ribs, cleft palate, and abnormal craniofacial ossification. These effects are consistent with the inhibition of estrogen synthesis in rats and may be a result of known effects of lowered estrogen on pregnancy, organogenesis, and parturition.
Fluconazole was present in low levels in breast milk following administration of a single 150 mg dose, based on data from a study in 10 breastfeeding women who temporarily or permanently discontinued breastfeeding 5 days to 19 months postpartum. The estimated daily infant dose of fluconazole from breast milk (assuming mean milk consumption of 150 mL/kg/day) based on the mean peak milk concentration (2.61 mcg/mL [range: 1.57 to 3.65 mcg/mL] at 5.2 hours post-dose) was 0.39 mg/kg/day, which is approximately 13% of the recommended pediatric dose for oropharyngeal candidiasis. (Labeled pediatric dose is 6 mg/kg/day on the first day followed by 3 mg/kg/day; estimated infant dose is 13% of 3 mg/kg/day maintenance dose). There are no data on fluconazole levels in milk after repeated use or after high-dose fluconazole. A published survey of 96 breastfeeding women who were treated with fluconazole 150 mg every other day (average of 7.3 capsules [range 1 to 29 capsules]) for lactation-associated candida of the breasts reported no serious adverse reactions in infants. Caution should be exercised when fluconazole is administered to a nursing woman.
An open-label, randomized, controlled trial has shown fluconazole to be effective in the treatment of oropharyngeal candidiasis in pediatric patients 6 months to 13 years of age. (See
The use of fluconazole in pediatric patients with
In a noncomparative study of fluconazole administered to pediatric patients (from birth to less than 17 years) with serious systemic fungal infections, most of which were candidemia, the effectiveness of fluconazole was similar to that reported for the treatment of candidemia in adults. Of 17 subjects with culture-confirmed candidemia, 11 of 14 (79%) with baseline symptoms (3 were asymptomatic) had a clinical cure; 13/15 (87%) of evaluable patients had a mycologic cure at the end of treatment but two of these patients relapsed at 10 and 18 days, respectively, following cessation of therapy.
The efficacy of fluconazole for the suppression of
The safety profile of fluconazole has been studied in 577 pediatric patients from 1 day to 17 years of age who received doses ranging from 1 to 15 mg/kg/day for 1 to 1,616 days. (See
A prospective, open-label, single-center study was conducted to determine the PK and safety of fluconazole in pediatric patients (ages: from birth to 17 years of age) on ECMO (see
Safety and effectiveness of fluconazole for the prophylaxis of invasive candidiasis in pediatric patients (premature infants weighing less than 750 grams at birth) have not been established.
A prospective, randomized, double-blind, placebo-controlled, multicenter trial was conducted in premature infants weighing less than 750 grams at birth to evaluate the efficacy and safety of prophylactic fluconazole 6 mg/kg administered twice weekly for 6 weeks versus placebo (NCT00734539). Efficacy was assessed using the endpoint of death or candidiasis by study day 49. The results are summarized in Table 4.
Fluconazole (N=188) n (%) | Placebo (N=173) n (%) | P-value | Difference (95% CI) | |
| Death or candidiasis* | 33 (17.6) | 38 (22.0) | 0.2954 | -4.4(-12.6, 3.8) |
| Components of endpoint** Death Candidiasis Missing | 27 (14.4) 6 (3.2) 2 (1.0) | 25 (14.5) 16 (9.2) 1 (0.5) |
* Subjects with missing data are imputed as having candidiasis or died.
**Subjects may be counted more than once as two fluconazole subjects and four placebo subjects diagnosed with candidiasis subsequently died by day 49.
The most common fatal serious adverse reactions in the fluconazole vs placebo arms, respectively, were necrotizing enterocolitis (NEC), 9 (5%) vs 9 (5%); neonatal bacterial sepsis, 6 (3%) vs 7 (4%); and neonatal respiratory failure, 4 (2%) vs 2 (0.6%).
The most common serious adverse reactions (>5%), reported in patients receiving fluconazole prophylaxis are displayed in Table 5.
Adverse Reaction | Fluconazole (N=188) n(%) | Placebo (N=173) n (%) |
| Necrotizing Enterocolitis (NEC) | 27 (14) | 28 (16) |
| Intestinal Perforation (includes ileal/small intestinal perforation) | 13 (7) | 7 (4) |
| Neonatal Respiratory Arrest/Neonatal Respiratory Failure | 13 (7) | 4 (2) |
| Bacterial Sepsis, Neonatal | 10 (5) | 12 (7) |
*All serious adverse reactions were assessed and recorded up through 30 days after the final dose of study drug. Serious adverse reactions included both fatal and non-fatal outcomes.
In non-AIDS patients, side effects possibly related to fluconazole treatment were reported in fewer patients aged 65 and older (9%, n=339) than for younger patients (14%, n=2240). However, there was no consistent difference between the older and younger patients with respect to individual side effects. Of the most frequently reported (>1%) side effects, rash, vomiting, and diarrhea occurred in greater proportions of older patients. Similar proportions of older patients (2.4%) and younger patients (1.5%) discontinued fluconazole therapy because of side effects. In post-marketing experience, spontaneous reports of anemia and acute renal failure were more frequent among patients 65 years of age or older than in those between 12 and 65 years of age. Because of the voluntary nature of the reports and the natural increase in the incidence of anemia and renal failure in the elderly, it is however not possible to establish a causal relationship to drug exposure.
Controlled clinical trials of fluconazole did not include sufficient numbers of patients aged 65 and older to evaluate whether they respond differently from younger patients in each indication. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Fluconazole is primarily cleared by renal excretion as unchanged drug. Because elderly patients are more likely to have decreased renal function, care should be taken to adjust dose based on creatinine clearance. It may be useful to monitor renal function. (See
Fluconazole is generally well tolerated.
In some patients, particularly those with serious underlying diseases such as AIDS and cancer, changes in renal and hematological function test results and hepatic abnormalities have been observed during treatment with fluconazole and comparative agents, but the clinical significance and relationship to treatment is uncertain.
Fluconazole for oral suspension is contraindicated in patients who have shown hypersensitivity to fluconazole or to any of its excipients. There is no information regarding cross-hypersensitivity between fluconazole and other azole antifungal agents. Caution should be used in prescribing fluconazole for oral suspension to patients with hypersensitivity to other azoles. Coadministration of other drugs known to prolong the QT interval and which are metabolized via the enzyme CYP3A4 such as erythromycin, pimozide, and quinidine are contraindicated in patients receiving fluconazole. (See
Abrocitinib
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmaxof 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmaxand AUC data from a food-effect study involving administration of fluconazole tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Dose regimen | Cmax (mcg/mL) | AUC0-24 (mcg*h/mL) | Half-life (hours) |
| 50 mg oral (once daily x 7 days) | 2.21 | 37.6 | 26.6 |
| 100 mg oral (once daily x 7 days) | 4.81 | 82.5 | 27.7 |
| 150 mg single oral | 2.70 | 137* | 34.1 |
| 200 mg oral (once daily x 14 days) | 10.12 | 169.5 | 31 |
| 300 mg oral (once daily x 14 days) | 15.98 | 299.4 | 34 |
| 400 mg oral (once daily x 14 days) | 18.89 | 349.9 | 31 |
*AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 to 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 to 0.7 |
*Relative to concurrent concentrations in plasma in subjects with normal renal function.
†Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Age Studied | Dose (mg/kg) | Clearance (mL/min/kg) | Half-life (Hours) | Cmax (mcg/mL) | AUC (mcg * h/mL) | Vdss (L/kg) |
| 2 to 60 days | IV 25 mg/kg on day one followed by IV 12 mg/kg once daily | 0.29 (35%) N=8 | 54.2 | 23.4 (29%) N=8 | 439 (25%)^ | 1.13 (31%) |
| 9 months to 13 years | Single-Oral 2 mg/kg | 0.4 (38%) N=14 | 25 | 2.9 (22%) N=16 | 94.7 (34%)* N=14 | N/A |
| 9 months to 13 years | Single-Oral 8 mg/kg | 0.51 (60%) N=15 | 19.5 | 9.8 (20%) N=15 | 362.5 (58%)* N=14 | N/A |
| 5 to 15 years | Multiple IV 2 mg/kg | 0.49 (40%) N=4 | 17.4 | 5.5 (25%) N=5 | 67.4 (26%)^ N=4 | 0.722 (36%) N=4 |
| 5 to 15 years | Multiple IV 4 mg/kg | 0.59 (64%) N=5 | 15.2 | 11.4 (44%) N=6 | 139.1 (46%)^ N=5 | 0.729 (33%) N=5 |
| 5 to 15 years | Multiple IV 8 mg/kg | 0.66 (31%) N=7 | 17.6 | 14.1 (22%) N=8 | 196.7 (25%)^ N=7 | 1.069 (37%) N=7 |
*Data for Clearance, Cmax, AUC and Vdssare presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.18 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24>400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.).
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmaxwas 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg⋅h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life-creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by
Resistance in
Antimicrobial
Fluconazole has been shown to be active against most isolates of the following microorganisms
The following
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
Alfentanil
Azithromycin
Coumarin-type anticoagulants:
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmaxof 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmaxand AUC data from a food-effect study involving administration of fluconazole tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Dose regimen | Cmax (mcg/mL) | AUC0-24 (mcg*h/mL) | Half-life (hours) |
| 50 mg oral (once daily x 7 days) | 2.21 | 37.6 | 26.6 |
| 100 mg oral (once daily x 7 days) | 4.81 | 82.5 | 27.7 |
| 150 mg single oral | 2.70 | 137* | 34.1 |
| 200 mg oral (once daily x 14 days) | 10.12 | 169.5 | 31 |
| 300 mg oral (once daily x 14 days) | 15.98 | 299.4 | 34 |
| 400 mg oral (once daily x 14 days) | 18.89 | 349.9 | 31 |
*AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 to 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 to 0.7 |
*Relative to concurrent concentrations in plasma in subjects with normal renal function.
†Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Age Studied | Dose (mg/kg) | Clearance (mL/min/kg) | Half-life (Hours) | Cmax (mcg/mL) | AUC (mcg * h/mL) | Vdss (L/kg) |
| 2 to 60 days | IV 25 mg/kg on day one followed by IV 12 mg/kg once daily | 0.29 (35%) N=8 | 54.2 | 23.4 (29%) N=8 | 439 (25%)^ | 1.13 (31%) |
| 9 months to 13 years | Single-Oral 2 mg/kg | 0.4 (38%) N=14 | 25 | 2.9 (22%) N=16 | 94.7 (34%)* N=14 | N/A |
| 9 months to 13 years | Single-Oral 8 mg/kg | 0.51 (60%) N=15 | 19.5 | 9.8 (20%) N=15 | 362.5 (58%)* N=14 | N/A |
| 5 to 15 years | Multiple IV 2 mg/kg | 0.49 (40%) N=4 | 17.4 | 5.5 (25%) N=5 | 67.4 (26%)^ N=4 | 0.722 (36%) N=4 |
| 5 to 15 years | Multiple IV 4 mg/kg | 0.59 (64%) N=5 | 15.2 | 11.4 (44%) N=6 | 139.1 (46%)^ N=5 | 0.729 (33%) N=5 |
| 5 to 15 years | Multiple IV 8 mg/kg | 0.66 (31%) N=7 | 17.6 | 14.1 (22%) N=8 | 196.7 (25%)^ N=7 | 1.069 (37%) N=7 |
*Data for Clearance, Cmax, AUC and Vdssare presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.18 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24>400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.).
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmaxwas 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg⋅h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life-creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by
Resistance in
Antimicrobial
Fluconazole has been shown to be active against most isolates of the following microorganisms
The following
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmaxof 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmaxand AUC data from a food-effect study involving administration of fluconazole tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Dose regimen | Cmax (mcg/mL) | AUC0-24 (mcg*h/mL) | Half-life (hours) |
| 50 mg oral (once daily x 7 days) | 2.21 | 37.6 | 26.6 |
| 100 mg oral (once daily x 7 days) | 4.81 | 82.5 | 27.7 |
| 150 mg single oral | 2.70 | 137* | 34.1 |
| 200 mg oral (once daily x 14 days) | 10.12 | 169.5 | 31 |
| 300 mg oral (once daily x 14 days) | 15.98 | 299.4 | 34 |
| 400 mg oral (once daily x 14 days) | 18.89 | 349.9 | 31 |
*AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 to 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 to 0.7 |
*Relative to concurrent concentrations in plasma in subjects with normal renal function.
†Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Age Studied | Dose (mg/kg) | Clearance (mL/min/kg) | Half-life (Hours) | Cmax (mcg/mL) | AUC (mcg * h/mL) | Vdss (L/kg) |
| 2 to 60 days | IV 25 mg/kg on day one followed by IV 12 mg/kg once daily | 0.29 (35%) N=8 | 54.2 | 23.4 (29%) N=8 | 439 (25%)^ | 1.13 (31%) |
| 9 months to 13 years | Single-Oral 2 mg/kg | 0.4 (38%) N=14 | 25 | 2.9 (22%) N=16 | 94.7 (34%)* N=14 | N/A |
| 9 months to 13 years | Single-Oral 8 mg/kg | 0.51 (60%) N=15 | 19.5 | 9.8 (20%) N=15 | 362.5 (58%)* N=14 | N/A |
| 5 to 15 years | Multiple IV 2 mg/kg | 0.49 (40%) N=4 | 17.4 | 5.5 (25%) N=5 | 67.4 (26%)^ N=4 | 0.722 (36%) N=4 |
| 5 to 15 years | Multiple IV 4 mg/kg | 0.59 (64%) N=5 | 15.2 | 11.4 (44%) N=6 | 139.1 (46%)^ N=5 | 0.729 (33%) N=5 |
| 5 to 15 years | Multiple IV 8 mg/kg | 0.66 (31%) N=7 | 17.6 | 14.1 (22%) N=8 | 196.7 (25%)^ N=7 | 1.069 (37%) N=7 |
*Data for Clearance, Cmax, AUC and Vdssare presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.18 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24>400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.).
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmaxwas 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg⋅h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life-creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by
Resistance in
Antimicrobial
Fluconazole has been shown to be active against most isolates of the following microorganisms
The following
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
HMG-CoA reductase inhibitors
Hydrochlorothiazide
Ibrutinib:
Ivacaftor and fixed dose ivacaftor combinations (e.g.,tezacaftor/ivacaftor and ivacaftor/tezacaftor/elexacaftor):
Lemborexant:
Losartan
Lurasidone:
Methadone
Although not specifically studied, fluconazole has the potential to increase the systemic exposure of other non-steroidal anti-inflammatory drugs (NSAIDs) that are metabolized by CYP2C9 (e.g., naproxen, lornoxicam, meloxicam, diclofenac). Frequent monitoring for adverse events and toxicity related to NSAIDs is recommended. Adjustment of dosage of NSAIDs may be needed.
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmaxof 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmaxand AUC data from a food-effect study involving administration of fluconazole tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Dose regimen | Cmax (mcg/mL) | AUC0-24 (mcg*h/mL) | Half-life (hours) |
| 50 mg oral (once daily x 7 days) | 2.21 | 37.6 | 26.6 |
| 100 mg oral (once daily x 7 days) | 4.81 | 82.5 | 27.7 |
| 150 mg single oral | 2.70 | 137* | 34.1 |
| 200 mg oral (once daily x 14 days) | 10.12 | 169.5 | 31 |
| 300 mg oral (once daily x 14 days) | 15.98 | 299.4 | 34 |
| 400 mg oral (once daily x 14 days) | 18.89 | 349.9 | 31 |
*AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 to 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 to 0.7 |
*Relative to concurrent concentrations in plasma in subjects with normal renal function.
†Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Age Studied | Dose (mg/kg) | Clearance (mL/min/kg) | Half-life (Hours) | Cmax (mcg/mL) | AUC (mcg * h/mL) | Vdss (L/kg) |
| 2 to 60 days | IV 25 mg/kg on day one followed by IV 12 mg/kg once daily | 0.29 (35%) N=8 | 54.2 | 23.4 (29%) N=8 | 439 (25%)^ | 1.13 (31%) |
| 9 months to 13 years | Single-Oral 2 mg/kg | 0.4 (38%) N=14 | 25 | 2.9 (22%) N=16 | 94.7 (34%)* N=14 | N/A |
| 9 months to 13 years | Single-Oral 8 mg/kg | 0.51 (60%) N=15 | 19.5 | 9.8 (20%) N=15 | 362.5 (58%)* N=14 | N/A |
| 5 to 15 years | Multiple IV 2 mg/kg | 0.49 (40%) N=4 | 17.4 | 5.5 (25%) N=5 | 67.4 (26%)^ N=4 | 0.722 (36%) N=4 |
| 5 to 15 years | Multiple IV 4 mg/kg | 0.59 (64%) N=5 | 15.2 | 11.4 (44%) N=6 | 139.1 (46%)^ N=5 | 0.729 (33%) N=5 |
| 5 to 15 years | Multiple IV 8 mg/kg | 0.66 (31%) N=7 | 17.6 | 14.1 (22%) N=8 | 196.7 (25%)^ N=7 | 1.069 (37%) N=7 |
*Data for Clearance, Cmax, AUC and Vdssare presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.18 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24>400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.).
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmaxwas 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg⋅h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life-creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by
Resistance in
Antimicrobial
Fluconazole has been shown to be active against most isolates of the following microorganisms
The following
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmaxof 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmaxand AUC data from a food-effect study involving administration of fluconazole tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Dose regimen | Cmax (mcg/mL) | AUC0-24 (mcg*h/mL) | Half-life (hours) |
| 50 mg oral (once daily x 7 days) | 2.21 | 37.6 | 26.6 |
| 100 mg oral (once daily x 7 days) | 4.81 | 82.5 | 27.7 |
| 150 mg single oral | 2.70 | 137* | 34.1 |
| 200 mg oral (once daily x 14 days) | 10.12 | 169.5 | 31 |
| 300 mg oral (once daily x 14 days) | 15.98 | 299.4 | 34 |
| 400 mg oral (once daily x 14 days) | 18.89 | 349.9 | 31 |
*AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 to 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 to 0.7 |
*Relative to concurrent concentrations in plasma in subjects with normal renal function.
†Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Age Studied | Dose (mg/kg) | Clearance (mL/min/kg) | Half-life (Hours) | Cmax (mcg/mL) | AUC (mcg * h/mL) | Vdss (L/kg) |
| 2 to 60 days | IV 25 mg/kg on day one followed by IV 12 mg/kg once daily | 0.29 (35%) N=8 | 54.2 | 23.4 (29%) N=8 | 439 (25%)^ | 1.13 (31%) |
| 9 months to 13 years | Single-Oral 2 mg/kg | 0.4 (38%) N=14 | 25 | 2.9 (22%) N=16 | 94.7 (34%)* N=14 | N/A |
| 9 months to 13 years | Single-Oral 8 mg/kg | 0.51 (60%) N=15 | 19.5 | 9.8 (20%) N=15 | 362.5 (58%)* N=14 | N/A |
| 5 to 15 years | Multiple IV 2 mg/kg | 0.49 (40%) N=4 | 17.4 | 5.5 (25%) N=5 | 67.4 (26%)^ N=4 | 0.722 (36%) N=4 |
| 5 to 15 years | Multiple IV 4 mg/kg | 0.59 (64%) N=5 | 15.2 | 11.4 (44%) N=6 | 139.1 (46%)^ N=5 | 0.729 (33%) N=5 |
| 5 to 15 years | Multiple IV 8 mg/kg | 0.66 (31%) N=7 | 17.6 | 14.1 (22%) N=8 | 196.7 (25%)^ N=7 | 1.069 (37%) N=7 |
*Data for Clearance, Cmax, AUC and Vdssare presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.18 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24>400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.).
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmaxwas 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg⋅h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life-creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by
Resistance in
Antimicrobial
Fluconazole has been shown to be active against most isolates of the following microorganisms
The following
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole for oral suspension is contraindicated in patients who have shown hypersensitivity to fluconazole or to any of its excipients. There is no information regarding cross-hypersensitivity between fluconazole and other azole antifungal agents. Caution should be used in prescribing fluconazole for oral suspension to patients with hypersensitivity to other azoles. Coadministration of other drugs known to prolong the QT interval and which are metabolized via the enzyme CYP3A4 such as erythromycin, pimozide, and quinidine are contraindicated in patients receiving fluconazole. (See
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmaxof 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmaxand AUC data from a food-effect study involving administration of fluconazole tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Dose regimen | Cmax (mcg/mL) | AUC0-24 (mcg*h/mL) | Half-life (hours) |
| 50 mg oral (once daily x 7 days) | 2.21 | 37.6 | 26.6 |
| 100 mg oral (once daily x 7 days) | 4.81 | 82.5 | 27.7 |
| 150 mg single oral | 2.70 | 137* | 34.1 |
| 200 mg oral (once daily x 14 days) | 10.12 | 169.5 | 31 |
| 300 mg oral (once daily x 14 days) | 15.98 | 299.4 | 34 |
| 400 mg oral (once daily x 14 days) | 18.89 | 349.9 | 31 |
*AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 to 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 to 0.7 |
*Relative to concurrent concentrations in plasma in subjects with normal renal function.
†Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Age Studied | Dose (mg/kg) | Clearance (mL/min/kg) | Half-life (Hours) | Cmax (mcg/mL) | AUC (mcg * h/mL) | Vdss (L/kg) |
| 2 to 60 days | IV 25 mg/kg on day one followed by IV 12 mg/kg once daily | 0.29 (35%) N=8 | 54.2 | 23.4 (29%) N=8 | 439 (25%)^ | 1.13 (31%) |
| 9 months to 13 years | Single-Oral 2 mg/kg | 0.4 (38%) N=14 | 25 | 2.9 (22%) N=16 | 94.7 (34%)* N=14 | N/A |
| 9 months to 13 years | Single-Oral 8 mg/kg | 0.51 (60%) N=15 | 19.5 | 9.8 (20%) N=15 | 362.5 (58%)* N=14 | N/A |
| 5 to 15 years | Multiple IV 2 mg/kg | 0.49 (40%) N=4 | 17.4 | 5.5 (25%) N=5 | 67.4 (26%)^ N=4 | 0.722 (36%) N=4 |
| 5 to 15 years | Multiple IV 4 mg/kg | 0.59 (64%) N=5 | 15.2 | 11.4 (44%) N=6 | 139.1 (46%)^ N=5 | 0.729 (33%) N=5 |
| 5 to 15 years | Multiple IV 8 mg/kg | 0.66 (31%) N=7 | 17.6 | 14.1 (22%) N=8 | 196.7 (25%)^ N=7 | 1.069 (37%) N=7 |
*Data for Clearance, Cmax, AUC and Vdssare presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.18 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24>400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.).
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmaxwas 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg⋅h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life-creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by
Resistance in
Antimicrobial
Fluconazole has been shown to be active against most isolates of the following microorganisms
The following
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmaxof 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmaxand AUC data from a food-effect study involving administration of fluconazole tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Dose regimen | Cmax (mcg/mL) | AUC0-24 (mcg*h/mL) | Half-life (hours) |
| 50 mg oral (once daily x 7 days) | 2.21 | 37.6 | 26.6 |
| 100 mg oral (once daily x 7 days) | 4.81 | 82.5 | 27.7 |
| 150 mg single oral | 2.70 | 137* | 34.1 |
| 200 mg oral (once daily x 14 days) | 10.12 | 169.5 | 31 |
| 300 mg oral (once daily x 14 days) | 15.98 | 299.4 | 34 |
| 400 mg oral (once daily x 14 days) | 18.89 | 349.9 | 31 |
*AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 to 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 to 0.7 |
*Relative to concurrent concentrations in plasma in subjects with normal renal function.
†Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Age Studied | Dose (mg/kg) | Clearance (mL/min/kg) | Half-life (Hours) | Cmax (mcg/mL) | AUC (mcg * h/mL) | Vdss (L/kg) |
| 2 to 60 days | IV 25 mg/kg on day one followed by IV 12 mg/kg once daily | 0.29 (35%) N=8 | 54.2 | 23.4 (29%) N=8 | 439 (25%)^ | 1.13 (31%) |
| 9 months to 13 years | Single-Oral 2 mg/kg | 0.4 (38%) N=14 | 25 | 2.9 (22%) N=16 | 94.7 (34%)* N=14 | N/A |
| 9 months to 13 years | Single-Oral 8 mg/kg | 0.51 (60%) N=15 | 19.5 | 9.8 (20%) N=15 | 362.5 (58%)* N=14 | N/A |
| 5 to 15 years | Multiple IV 2 mg/kg | 0.49 (40%) N=4 | 17.4 | 5.5 (25%) N=5 | 67.4 (26%)^ N=4 | 0.722 (36%) N=4 |
| 5 to 15 years | Multiple IV 4 mg/kg | 0.59 (64%) N=5 | 15.2 | 11.4 (44%) N=6 | 139.1 (46%)^ N=5 | 0.729 (33%) N=5 |
| 5 to 15 years | Multiple IV 8 mg/kg | 0.66 (31%) N=7 | 17.6 | 14.1 (22%) N=8 | 196.7 (25%)^ N=7 | 1.069 (37%) N=7 |
*Data for Clearance, Cmax, AUC and Vdssare presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.18 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24>400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.).
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmaxwas 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg⋅h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life-creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by
Resistance in
Antimicrobial
Fluconazole has been shown to be active against most isolates of the following microorganisms
The following
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmaxof 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmaxand AUC data from a food-effect study involving administration of fluconazole tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Dose regimen | Cmax (mcg/mL) | AUC0-24 (mcg*h/mL) | Half-life (hours) |
| 50 mg oral (once daily x 7 days) | 2.21 | 37.6 | 26.6 |
| 100 mg oral (once daily x 7 days) | 4.81 | 82.5 | 27.7 |
| 150 mg single oral | 2.70 | 137* | 34.1 |
| 200 mg oral (once daily x 14 days) | 10.12 | 169.5 | 31 |
| 300 mg oral (once daily x 14 days) | 15.98 | 299.4 | 34 |
| 400 mg oral (once daily x 14 days) | 18.89 | 349.9 | 31 |
*AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 to 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 to 0.7 |
*Relative to concurrent concentrations in plasma in subjects with normal renal function.
†Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Age Studied | Dose (mg/kg) | Clearance (mL/min/kg) | Half-life (Hours) | Cmax (mcg/mL) | AUC (mcg * h/mL) | Vdss (L/kg) |
| 2 to 60 days | IV 25 mg/kg on day one followed by IV 12 mg/kg once daily | 0.29 (35%) N=8 | 54.2 | 23.4 (29%) N=8 | 439 (25%)^ | 1.13 (31%) |
| 9 months to 13 years | Single-Oral 2 mg/kg | 0.4 (38%) N=14 | 25 | 2.9 (22%) N=16 | 94.7 (34%)* N=14 | N/A |
| 9 months to 13 years | Single-Oral 8 mg/kg | 0.51 (60%) N=15 | 19.5 | 9.8 (20%) N=15 | 362.5 (58%)* N=14 | N/A |
| 5 to 15 years | Multiple IV 2 mg/kg | 0.49 (40%) N=4 | 17.4 | 5.5 (25%) N=5 | 67.4 (26%)^ N=4 | 0.722 (36%) N=4 |
| 5 to 15 years | Multiple IV 4 mg/kg | 0.59 (64%) N=5 | 15.2 | 11.4 (44%) N=6 | 139.1 (46%)^ N=5 | 0.729 (33%) N=5 |
| 5 to 15 years | Multiple IV 8 mg/kg | 0.66 (31%) N=7 | 17.6 | 14.1 (22%) N=8 | 196.7 (25%)^ N=7 | 1.069 (37%) N=7 |
*Data for Clearance, Cmax, AUC and Vdssare presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.18 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24>400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.).
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmaxwas 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg⋅h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life-creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by
Resistance in
Antimicrobial
Fluconazole has been shown to be active against most isolates of the following microorganisms
The following
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmaxof 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmaxand AUC data from a food-effect study involving administration of fluconazole tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Dose regimen | Cmax (mcg/mL) | AUC0-24 (mcg*h/mL) | Half-life (hours) |
| 50 mg oral (once daily x 7 days) | 2.21 | 37.6 | 26.6 |
| 100 mg oral (once daily x 7 days) | 4.81 | 82.5 | 27.7 |
| 150 mg single oral | 2.70 | 137* | 34.1 |
| 200 mg oral (once daily x 14 days) | 10.12 | 169.5 | 31 |
| 300 mg oral (once daily x 14 days) | 15.98 | 299.4 | 34 |
| 400 mg oral (once daily x 14 days) | 18.89 | 349.9 | 31 |
*AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 to 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 to 0.7 |
*Relative to concurrent concentrations in plasma in subjects with normal renal function.
†Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Age Studied | Dose (mg/kg) | Clearance (mL/min/kg) | Half-life (Hours) | Cmax (mcg/mL) | AUC (mcg * h/mL) | Vdss (L/kg) |
| 2 to 60 days | IV 25 mg/kg on day one followed by IV 12 mg/kg once daily | 0.29 (35%) N=8 | 54.2 | 23.4 (29%) N=8 | 439 (25%)^ | 1.13 (31%) |
| 9 months to 13 years | Single-Oral 2 mg/kg | 0.4 (38%) N=14 | 25 | 2.9 (22%) N=16 | 94.7 (34%)* N=14 | N/A |
| 9 months to 13 years | Single-Oral 8 mg/kg | 0.51 (60%) N=15 | 19.5 | 9.8 (20%) N=15 | 362.5 (58%)* N=14 | N/A |
| 5 to 15 years | Multiple IV 2 mg/kg | 0.49 (40%) N=4 | 17.4 | 5.5 (25%) N=5 | 67.4 (26%)^ N=4 | 0.722 (36%) N=4 |
| 5 to 15 years | Multiple IV 4 mg/kg | 0.59 (64%) N=5 | 15.2 | 11.4 (44%) N=6 | 139.1 (46%)^ N=5 | 0.729 (33%) N=5 |
| 5 to 15 years | Multiple IV 8 mg/kg | 0.66 (31%) N=7 | 17.6 | 14.1 (22%) N=8 | 196.7 (25%)^ N=7 | 1.069 (37%) N=7 |
*Data for Clearance, Cmax, AUC and Vdssare presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.18 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24>400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.).
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmaxwas 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg⋅h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life-creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by
Resistance in
Antimicrobial
Fluconazole has been shown to be active against most isolates of the following microorganisms
The following
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Theophylline:
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmaxof 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmaxand AUC data from a food-effect study involving administration of fluconazole tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Dose regimen | Cmax (mcg/mL) | AUC0-24 (mcg*h/mL) | Half-life (hours) |
| 50 mg oral (once daily x 7 days) | 2.21 | 37.6 | 26.6 |
| 100 mg oral (once daily x 7 days) | 4.81 | 82.5 | 27.7 |
| 150 mg single oral | 2.70 | 137* | 34.1 |
| 200 mg oral (once daily x 14 days) | 10.12 | 169.5 | 31 |
| 300 mg oral (once daily x 14 days) | 15.98 | 299.4 | 34 |
| 400 mg oral (once daily x 14 days) | 18.89 | 349.9 | 31 |
*AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 to 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 to 0.7 |
*Relative to concurrent concentrations in plasma in subjects with normal renal function.
†Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Age Studied | Dose (mg/kg) | Clearance (mL/min/kg) | Half-life (Hours) | Cmax (mcg/mL) | AUC (mcg * h/mL) | Vdss (L/kg) |
| 2 to 60 days | IV 25 mg/kg on day one followed by IV 12 mg/kg once daily | 0.29 (35%) N=8 | 54.2 | 23.4 (29%) N=8 | 439 (25%)^ | 1.13 (31%) |
| 9 months to 13 years | Single-Oral 2 mg/kg | 0.4 (38%) N=14 | 25 | 2.9 (22%) N=16 | 94.7 (34%)* N=14 | N/A |
| 9 months to 13 years | Single-Oral 8 mg/kg | 0.51 (60%) N=15 | 19.5 | 9.8 (20%) N=15 | 362.5 (58%)* N=14 | N/A |
| 5 to 15 years | Multiple IV 2 mg/kg | 0.49 (40%) N=4 | 17.4 | 5.5 (25%) N=5 | 67.4 (26%)^ N=4 | 0.722 (36%) N=4 |
| 5 to 15 years | Multiple IV 4 mg/kg | 0.59 (64%) N=5 | 15.2 | 11.4 (44%) N=6 | 139.1 (46%)^ N=5 | 0.729 (33%) N=5 |
| 5 to 15 years | Multiple IV 8 mg/kg | 0.66 (31%) N=7 | 17.6 | 14.1 (22%) N=8 | 196.7 (25%)^ N=7 | 1.069 (37%) N=7 |
*Data for Clearance, Cmax, AUC and Vdssare presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.18 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24>400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.).
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmaxwas 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg⋅h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life-creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by
Resistance in
Antimicrobial
Fluconazole has been shown to be active against most isolates of the following microorganisms
The following
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmaxof 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmaxand AUC data from a food-effect study involving administration of fluconazole tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Dose regimen | Cmax (mcg/mL) | AUC0-24 (mcg*h/mL) | Half-life (hours) |
| 50 mg oral (once daily x 7 days) | 2.21 | 37.6 | 26.6 |
| 100 mg oral (once daily x 7 days) | 4.81 | 82.5 | 27.7 |
| 150 mg single oral | 2.70 | 137* | 34.1 |
| 200 mg oral (once daily x 14 days) | 10.12 | 169.5 | 31 |
| 300 mg oral (once daily x 14 days) | 15.98 | 299.4 | 34 |
| 400 mg oral (once daily x 14 days) | 18.89 | 349.9 | 31 |
*AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 to 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 to 0.7 |
*Relative to concurrent concentrations in plasma in subjects with normal renal function.
†Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Age Studied | Dose (mg/kg) | Clearance (mL/min/kg) | Half-life (Hours) | Cmax (mcg/mL) | AUC (mcg * h/mL) | Vdss (L/kg) |
| 2 to 60 days | IV 25 mg/kg on day one followed by IV 12 mg/kg once daily | 0.29 (35%) N=8 | 54.2 | 23.4 (29%) N=8 | 439 (25%)^ | 1.13 (31%) |
| 9 months to 13 years | Single-Oral 2 mg/kg | 0.4 (38%) N=14 | 25 | 2.9 (22%) N=16 | 94.7 (34%)* N=14 | N/A |
| 9 months to 13 years | Single-Oral 8 mg/kg | 0.51 (60%) N=15 | 19.5 | 9.8 (20%) N=15 | 362.5 (58%)* N=14 | N/A |
| 5 to 15 years | Multiple IV 2 mg/kg | 0.49 (40%) N=4 | 17.4 | 5.5 (25%) N=5 | 67.4 (26%)^ N=4 | 0.722 (36%) N=4 |
| 5 to 15 years | Multiple IV 4 mg/kg | 0.59 (64%) N=5 | 15.2 | 11.4 (44%) N=6 | 139.1 (46%)^ N=5 | 0.729 (33%) N=5 |
| 5 to 15 years | Multiple IV 8 mg/kg | 0.66 (31%) N=7 | 17.6 | 14.1 (22%) N=8 | 196.7 (25%)^ N=7 | 1.069 (37%) N=7 |
*Data for Clearance, Cmax, AUC and Vdssare presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.18 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24>400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.).
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmaxwas 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg⋅h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life-creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by
Resistance in
Antimicrobial
Fluconazole has been shown to be active against most isolates of the following microorganisms
The following
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Triazolam:
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmaxof 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmaxand AUC data from a food-effect study involving administration of fluconazole tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Dose regimen | Cmax (mcg/mL) | AUC0-24 (mcg*h/mL) | Half-life (hours) |
| 50 mg oral (once daily x 7 days) | 2.21 | 37.6 | 26.6 |
| 100 mg oral (once daily x 7 days) | 4.81 | 82.5 | 27.7 |
| 150 mg single oral | 2.70 | 137* | 34.1 |
| 200 mg oral (once daily x 14 days) | 10.12 | 169.5 | 31 |
| 300 mg oral (once daily x 14 days) | 15.98 | 299.4 | 34 |
| 400 mg oral (once daily x 14 days) | 18.89 | 349.9 | 31 |
*AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 to 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 to 0.7 |
*Relative to concurrent concentrations in plasma in subjects with normal renal function.
†Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Age Studied | Dose (mg/kg) | Clearance (mL/min/kg) | Half-life (Hours) | Cmax (mcg/mL) | AUC (mcg * h/mL) | Vdss (L/kg) |
| 2 to 60 days | IV 25 mg/kg on day one followed by IV 12 mg/kg once daily | 0.29 (35%) N=8 | 54.2 | 23.4 (29%) N=8 | 439 (25%)^ | 1.13 (31%) |
| 9 months to 13 years | Single-Oral 2 mg/kg | 0.4 (38%) N=14 | 25 | 2.9 (22%) N=16 | 94.7 (34%)* N=14 | N/A |
| 9 months to 13 years | Single-Oral 8 mg/kg | 0.51 (60%) N=15 | 19.5 | 9.8 (20%) N=15 | 362.5 (58%)* N=14 | N/A |
| 5 to 15 years | Multiple IV 2 mg/kg | 0.49 (40%) N=4 | 17.4 | 5.5 (25%) N=5 | 67.4 (26%)^ N=4 | 0.722 (36%) N=4 |
| 5 to 15 years | Multiple IV 4 mg/kg | 0.59 (64%) N=5 | 15.2 | 11.4 (44%) N=6 | 139.1 (46%)^ N=5 | 0.729 (33%) N=5 |
| 5 to 15 years | Multiple IV 8 mg/kg | 0.66 (31%) N=7 | 17.6 | 14.1 (22%) N=8 | 196.7 (25%)^ N=7 | 1.069 (37%) N=7 |
*Data for Clearance, Cmax, AUC and Vdssare presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.18 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24>400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.).
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmaxwas 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg⋅h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life-creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by
Resistance in
Antimicrobial
Fluconazole has been shown to be active against most isolates of the following microorganisms
The following
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Physicians should be aware that interaction studies with medications other than those listed in the
The pharmacokinetic properties (PK) of fluconazole are similar following administration by the intravenous or oral routes. In normal volunteers, the bioavailability of orally administered fluconazole is over 90% compared with intravenous administration. Bioequivalence was established between the 100 mg tablet and both suspension strengths when administered as a single 200 mg dose.
Peak plasma concentrations (Cmax) in fasted normal volunteers occur between 1 and 2 hours with a terminal plasma elimination half-life of approximately 30 hours (range: 20 to 50 hours) after oral administration.
In fasted normal volunteers, administration of a single oral 400 mg dose of fluconazole leads to a mean Cmaxof 6.72 mcg/mL (range: 4.12 to 8.08 mcg/mL) and after single or multiple oral doses of 50 to 400 mg, fluconazole plasma concentrations and area under the plasma concentration time curve (AUC) are dose proportional (Table 1).
The Cmaxand AUC data from a food-effect study involving administration of fluconazole tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, fluconazole may be taken without regard to meals. (See DOSAGE AND ADMINISTRATION.)
Dose regimen | Cmax (mcg/mL) | AUC0-24 (mcg*h/mL) | Half-life (hours) |
| 50 mg oral (once daily x 7 days) | 2.21 | 37.6 | 26.6 |
| 100 mg oral (once daily x 7 days) | 4.81 | 82.5 | 27.7 |
| 150 mg single oral | 2.70 | 137* | 34.1 |
| 200 mg oral (once daily x 14 days) | 10.12 | 169.5 | 31 |
| 300 mg oral (once daily x 14 days) | 15.98 | 299.4 | 34 |
| 400 mg oral (once daily x 14 days) | 18.89 | 349.9 | 31 |
*AUC0-inf. Cmax= peak plasma concentrations, AUC =area under the plasma concentration time curve.
Steady-state concentrations are reached within 5 to 10 days following oral doses of 50 to 400 mg given once daily. Administration of a loading dose (on Day 1) of twice the usual daily dose results in plasma concentrations close to steady-state by the second day. The apparent volume of distribution of fluconazole approximates that of total body water. Plasma protein binding is low (11 to 12%). Following either single- or multiple oral doses for up to 14 days, fluconazole penetrates into all body fluids studied (see table below). In normal volunteers, saliva concentrations of fluconazole were equal to or slightly greater than plasma concentrations regardless of dose, route, or duration of dosing. In patients with bronchiectasis, sputum concentrations of fluconazole following a single 150 mg oral dose were equal to plasma concentrations at both 4 and 24 hours post dose. In patients with fungal meningitis, fluconazole concentrations in the cerebrospinal fluid (CSF) are approximately 80% of the corresponding plasma concentrations.
A single oral 150 mg dose of fluconazole administered to 27 patients penetrated into vaginal tissue, resulting in tissue: plasma ratios ranging from 0.94 to 1.14 over the first 48 hours following dosing.
A single oral 150 mg dose of fluconazole administered to 14 patients penetrated into vaginal fluid, resulting in fluid: plasma ratios ranging from 0.36 to 0.71 over the first 72 hours following dosing.
Tissue or Fluid | Ratio of Fluconazole Tissue (Fluid)/Plasma Concentration* |
| Cerebrospinal fluid† | 0.5 to 0.9 |
| Saliva | 1 |
| Sputum | 1 |
| Blister fluid | 1 |
| Urine | 10 |
| Normal skin | 10 |
| Nails | 1 |
| Blister skin | 2 |
| Vaginal tissue | 1 |
| Vaginal fluid | 0.4 to 0.7 |
*Relative to concurrent concentrations in plasma in subjects with normal renal function.
†Independent of degree of meningeal inflammation.
Mean body clearance in adults is reported to be 0.23 (17%) mL/min/kg. In normal volunteers, fluconazole is cleared primarily by renal excretion, with approximately 80% of the administered dose appearing in the urine as unchanged drug. About 11% of the dose is excreted in the urine as metabolites.
The pharmacokinetics of fluconazole are markedly affected by reduction in renal function. There is an inverse relationship between the elimination half-life and creatinine clearance. The dose of fluconazole may need to be reduced in patients with impaired renal function. (See
In normal volunteers, fluconazole administration (doses ranging from 200 mg to 400 mg once daily for up to 14 days) was associated with small and inconsistent effects on testosterone concentrations, endogenous corticosteroid concentrations, and the adrenocorticotropic hormone (ACTH)-stimulated cortisol response.
In pediatric patients from 2 days to 15 years of age, the following pharmacokinetic data have been reported following the administration of fluconazole:
Age Studied | Dose (mg/kg) | Clearance (mL/min/kg) | Half-life (Hours) | Cmax (mcg/mL) | AUC (mcg * h/mL) | Vdss (L/kg) |
| 2 to 60 days | IV 25 mg/kg on day one followed by IV 12 mg/kg once daily | 0.29 (35%) N=8 | 54.2 | 23.4 (29%) N=8 | 439 (25%)^ | 1.13 (31%) |
| 9 months to 13 years | Single-Oral 2 mg/kg | 0.4 (38%) N=14 | 25 | 2.9 (22%) N=16 | 94.7 (34%)* N=14 | N/A |
| 9 months to 13 years | Single-Oral 8 mg/kg | 0.51 (60%) N=15 | 19.5 | 9.8 (20%) N=15 | 362.5 (58%)* N=14 | N/A |
| 5 to 15 years | Multiple IV 2 mg/kg | 0.49 (40%) N=4 | 17.4 | 5.5 (25%) N=5 | 67.4 (26%)^ N=4 | 0.722 (36%) N=4 |
| 5 to 15 years | Multiple IV 4 mg/kg | 0.59 (64%) N=5 | 15.2 | 11.4 (44%) N=6 | 139.1 (46%)^ N=5 | 0.729 (33%) N=5 |
| 5 to 15 years | Multiple IV 8 mg/kg | 0.66 (31%) N=7 | 17.6 | 14.1 (22%) N=8 | 196.7 (25%)^ N=7 | 1.069 (37%) N=7 |
*Data for Clearance, Cmax, AUC and Vdssare presented as arithmetic mean (CV%) and Half-life as arithmetic mean only. *AUC0-inf; ^AUC0-24;
Abbreviations: Cmax=Peak plasma concentrations, AUC =area under the plasma concentration time curve; and Vdss=volume of distribution at steady state.
There are limited data available in patients 61 days to less than 9 months of age.
In pediatric patients (premature newborns; gestational age 26 to 29 weeks and postnatal age from birth to 1 day), the mean (%cv) clearance within 36 hours of birth was 0.18 (35%, N=7) mL/min/kg, which increased with time to a mean of 0.218 (31%, N=9) mL/min/kg 6 days later and 0.333 (56%, N=4) mL/min/kg 12 days later. Similarly, the half-life was 73.6 hours, which decreased with time to a mean of 53.2 hours 6 days later and 46.6 hours 12 days later.
In a study of 13 pediatric patients (preterm and term infants with median gestational age of 37 weeks, GA range 24 to 39 weeks; median postnatal age [PNA] 19 days, PNA range: 5 to 262 days) 12 infants received a 25 mg/kg loading dose, and 9/12 (75%) achieved an AUC0-24of >400 mg*h/L in the first 24 hours. A population pharmacokinetic model using data from 55 pediatric patients (GA 23 to 40 weeks, PNA 1 to 88 days) found that a loading dose of 25 mg/kg is necessary to reach target AUC >400 mg*h/L within 24 hours of initiating therapy in pediatric patients younger than 3 months of age. A maintenance dose of 9 mg/kg daily should be used in pediatric patients born at GA less than 30 weeks and 12 mg/kg daily in pediatric patients with GA equal or greater than 30 weeks. (See DOSAGE AND ADMINISTRATION.)
A population PK model using data from 21 pediatric patients ages from birth to 17 years supported with extracorporeal membrane oxygenation (ECMO), and 19 pediatric non-ECMO patients ages from birth to 2 years found that clearance was related to serum creatinine while a higher volume of distribution was related to presence of ECMO support. The median volume of distribution was 1.3 L/kg in pediatric patients on ECMO and 0.9 L/kg in those not on ECMO. Simulations suggested that a loading dose of 35 mg/kg is needed to achieve the target AUC0-24>400 mg*h/L within the first 24 hours in pediatric patients on ECMO. (See DOSAGE AND ADMINISTRATION.).
A pharmacokinetic study was conducted in 22 subjects, 65 years of age or older receiving a single 50 mg oral dose of fluconazole. Ten of these patients were concomitantly receiving diuretics. The Cmaxwas 1.54 mcg/mL and occurred at 1.3 hours post dose. The mean AUC was 76.4 ± 20.3 mcg⋅h/mL, and the mean terminal half-life was 46.2 hours. These pharmacokinetic parameter values are higher than analogous values reported for normal young male volunteers. Coadministration of diuretics did not significantly alter the AUC or Cmax. In addition, creatinine clearance (74 mL/min), the percent of drug recovered unchanged in urine (0 to 24 hours, 22%), and the fluconazole renal clearance estimates (0.124 mL/min/kg) for the elderly were generally lower than those of younger volunteers. Thus, the alteration of fluconazole disposition in the elderly appears to be related to reduced renal function characteristic of this group. A plot of each subject’s terminal elimination half-life versus creatinine clearance compared to the predicted half-life – creatinine clearance curve derived from normal subjects and subjects with varying degrees of renal insufficiency indicated that 21 of 22 subjects fell within the 95% confidence limit of the predicted half-life-creatinine clearance curves. These results are consistent with the hypothesis that higher values for the pharmacokinetic parameters observed in the elderly subjects compared to normal young male volunteers are due to the decreased kidney function that is expected in the elderly.
In a second study, twenty-five normal females received daily doses of both 200 mg fluconazole tablets or placebo for two, ten-day periods. The treatment cycles were one month apart with all subjects receiving fluconazole during one cycle and placebo during the other. The order of study treatment was random. Single doses of an oral contraceptive tablet containing levonorgestrel and ethinyl estradiol were administered on the final treatment day (Day 10) of both cycles. Following administration of 200 mg of fluconazole, the mean percentage increase of AUC for levonorgestrel compared to placebo was 25% (range: –12 to 82%) and the mean percentage increase for ethinyl estradiol compared to placebo was 38% (range: –11 to 101%). Both of these increases were statistically significantly different from placebo.
A third study evaluated the potential interaction of once-weekly dosing of fluconazole 300 mg to 21 normal females taking an oral contraceptive containing ethinyl estradiol and norethindrone. In this placebo-controlled, double-blind, randomized, two-way crossover study carried out over three cycles of oral contraceptive treatment, fluconazole dosing resulted in small increases in the mean AUCs of ethinyl estradiol and norethindrone compared to similar placebo dosing. The mean AUCs of ethinyl estradiol and norethindrone increased by 24% (95% C.I. range: 18 to 31%) and 13% (95% C.I. range: 8 to 18%), respectively, relative to placebo. Fluconazole treatment did not cause a decrease in the ethinyl estradiol AUC of any individual subject in this study compared to placebo dosing. The individual AUC values of norethindrone decreased very slightly (<5%) in 3 of the 21 subjects after fluconazole treatment.
Warfarin:
Phenytoin:
Quinidine:
Oral hypoglycemics:
Midazolam:
A second randomized, double-dummy, placebo-controlled, cross over study in three phases was performed to determine the effect of route of administration of fluconazole on the interaction between fluconazole and midazolam. In each phase the subjects were given oral fluconazole 400 mg and intravenous saline; oral placebo and intravenous fluconazole 400 mg; and oral placebo and IV saline. An oral dose of 7.5 mg of midazolam was ingested after fluconazole/placebo. The AUC and Cmaxof midazolam were significantly higher after oral than IV administration of fluconazole. Oral fluconazole increased the midazolam AUC and Cmaxby 272% and 129%, respectively. IV fluconazole increased the midazolam AUC and Cmaxby 244% and 79%, respectively. Both oral and IV fluconazole increased the pharmacodynamic effects of midazolam. (See
Azithromycin:
Abrocitinib
Fluconazole is a highly selective inhibitor of fungal cytochrome P450 dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol. The subsequent loss of normal sterols correlates with the accumulation of 14-α-methyl sterols in fungi and may be responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition.
Resistance
A potential for development of resistance to fluconazole is well known. Fungal isolates exhibiting reduced susceptibility to other azoles may also show reduced susceptibility to fluconazole. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fluconazole resistance may arise from a modification in the quality or quantity of the target enzyme (lanosterol 14-α-demethylase), reduced access to the drug target, or some combination of these mechanisms.
Point mutations in the gene (
The second major mechanism of drug resistance involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters; the major facilitators (encoded by
Resistance in
Antimicrobial
Fluconazole has been shown to be active against most isolates of the following microorganisms
The following
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
Fluconazole, the first of a new subclass of synthetic triazole antifungal agents, is available as a powder for oral suspension.
Fluconazole is designated chemically as 2,4-difluoro-α,α1-bis(1H-1,2,4-triazol-1-ylmethyl) benzyl alcohol with an empirical formula of C13H12F2N6O and molecular weight of 306.3. The structural formula is:
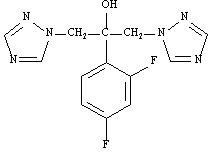
Fluconazole USP is a white or almost white, crystalline powder which is slightly soluble in water and saline.
Fluconazole for oral suspension USP contains 350 mg or 1400 mg of fluconazole USP and the following inactive ingredients: sodium benzoate, citric acid anhydrous, sodium citrate, xanthan gum, titanium dioxide, natural orange flavor, colloidal silicon dioxide, and sucrose. After reconstitution with 24 mL of distilled water or Purified Water (USP), each mL of reconstituted suspension contains 10 mg or 40 mg of fluconazole.