Fludrocortisone Acetate
Fludrocortisone Acetate Prescribing Information
Fludrocortisone acetate tablets, 0.1 mg are indicated as partial replacement therapy for primary and secondary adrenocortical insufficiency in Addison’s disease and for the treatment of salt-losing adrenogenital syndrome.
Dosage depends on the severity of the disease and the response of the patient. Patients should be continually monitored for signs that indicate dosage adjustment is necessary, such as remission or exacerbations of the disease and stress (surgery, infection, trauma) (see
In Addison’s disease, the combination of fludrocortisone acetate tablets with a glucocorticoid such as hydrocortisone or cortisone provides substitution therapy approximating normal adrenal activity with minimal risks of unwanted effects.
The usual dose is 0.1 mg of fludrocortisone acetate tablets daily, although dosage ranging from 0.1 mg three times a week to 0.2 mg daily has been employed. In the event transient hypertension develops as a consequence of therapy, the dose should be reduced to 0.05 mg daily. Fludrocortisone acetate tablets are preferably administered in conjunction with cortisone (10 mg to 37.5 mg daily in divided doses) or hydrocortisone (10 mg to 30 mg daily in divided doses).
The recommended dosage for treating the salt-losing adrenogenital syndrome is 0.1 mg to 0.2 mg of fludrocortisone acetate tablets daily.
Corticosteroids are contraindicated in patients with systemic fungal infections and in those with a history of possible or known hypersensitivity to these agents.
Most adverse reactions are caused by the drug’s mineralocorticoid activity (retention of sodium and water) and include hypertension, edema, cardiac enlargement, congestive heart failure, potassium loss, and hypokalemic alkalosis.
When fludrocortisone is used in the small dosages recommended, the glucocorticoid side effects often seen with cortisone and its derivatives are not usually a problem; however, the following untoward effects should be kept in mind, particularly when fludrocortisone is used over a prolonged period of time or in conjunction with cortisone or a similar glucocorticoid.
Other adverse reactions that may occur following the administration of a corticosteroid are necrotizing angiitis, thrombophlebitis, aggravation or masking of infections, insomnia, syncopal episodes, and anaphylactoid reactions.
When administered concurrently, the following drugs may interact with adrenal corticosteroids.
Fludrocortisone acetate tablets USP, 0.1 mg contain fludrocortisone acetate, a synthetic adrenocortical steroid possessing very potent mineralocorticoid properties and high glucocorticoid activity; it is used only for its mineralocorticoid effects. The chemical name for fludrocortisone acetate is 9-fluoro-11β, 17, 21-trihydroxypregn-4-ene-3, 20-dione 21-acetate; its structural formula is:
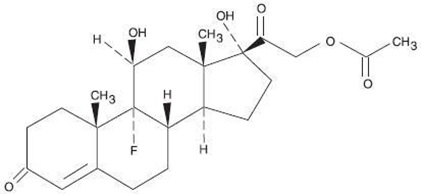
C
23H
31FO
6 MW 422.49
Fludrocortisone acetate tablets USP, 0.1 mg are available for oral administration as scored tablets providing 0.1 mg fludrocortisone acetate per tablet. Inactive ingredients: lactose anhydrous, lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, magnesium stearate.