Fluorodopa
(Fluorodopa F18)Fluorodopa Prescribing Information
Fluorodopa F 18 Injection is indicated for use in positron emission tomography (PET) to visualize dopaminergic nerve terminals in the striatum for the evaluation of adult patients with suspected Parkinsonian syndromes (PS). Fluorodopa F 18 PET is an adjunct to other diagnostic evaluations.
Fluorodopa F 18 PET scans are interpreted visually, based upon the appearance and shape of the putamen and caudate of the striatum. Optimum presentation of the reconstructed images for visual interpretation is transaxial slices parallel to the anterior commissure-posterior commissure (AC-PC) line. Determination of whether an image is negative or positive is made by assessing the shape and intensity of the striatal signal (see Figure 1 and 2). Image interpretation does not involve integration of the image with clinical signs and/or symptoms.
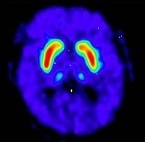
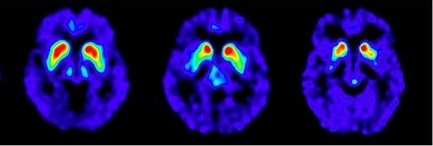
• FDOPA uptake is asymmetric in the putamen; normal on one side but reduced on the contralateral side with respect to the background, especially in the posterior part. Caudate uptake is symmetrical on both sides and clearly delineated from the background (see Figure 2, A).
• FDOPA uptake is bilaterally reduced in the putamen (see Figure 2, B).
• FDOPA uptake is bilaterally reduced in the putamen and in the caudate nuclei (see Figure 2, C).
Injection: clear, colorless solution containing 37 MBq/mL to 1,480 MBq/mL (1 mCi/mL to 40 mCi/mL) of Fluorodopa F 18 Injection, at calibration time in a multiple-dose glass vial.
The safety and effectiveness of Fluorodopa F 18 Injection for visualization of dopaminergic neurons in the striatum has not been established in pediatric patients.
None
Fluorodopa F 18 Injection use contributes to a patient’s overall long-term radiation exposure, which is associated with an increased risk of cancer. Use the smallest dose necessary for imaging and ensure safe handling to protect the patient and health care worker
• Handle Fluorodopa F 18 Injection with appropriate safety measures to minimize radiation exposure
• The recommended dose for adults is 185 megabecquerels (MBq) [5 millicuries (mCi)] administered as an intravenous injection infused over 1 minute.
• Minimize the dose of Fluorodopa F 18 Injection consistent with the objectives of the procedure and the nature of the imaging cameras employed.
• Use aseptic techniques and radiation shielding during all operations involved in the manipulation and administration of Fluorodopa F 18 Injection.
• Calculate the necessary volume to administer based on calibration time and dose.
• Inspect Fluorodopa F 18 Injection visually and do not use the drug if the solution contains particulate matter or is discolored.
• Measure the patient dose immediately prior to administration in a dose calibrator.
• Dispose of unused drug in compliance with applicable regulations.