Fluphenazine Hydrochloride Prescribing Information
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical, antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Fluphenazine Hydrochloride Injection, USP is not approved for the treatment of patients with dementia-related psychosis (see
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Fluphenazine Hydrochloride Injection, USP is not approved for the treatment of patients with dementia-related psychosis (see
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with neuroleptic (antipsychotic) drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of neuroleptic treatment, which patients are likely to develop the syndrome. Whether neuroleptic drug products differ in their potential to cause tardive dyskinesia is unknown.
Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of neuroleptic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if neuroleptic treatment is withdrawn. Neuroleptic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying disease process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, neuroleptics should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic neuroleptic treatment should generally be reserved for patients who suffer from a chronic illness that 1) is known to respond to neuroleptic drugs and 2) for whom alternative, equally effective, but potentially less harmful treatments are
If signs and symptoms of tardive dyskinesia appear in a patient on neuroleptics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome.
(For further information about the description of tardive dyskinesia and its clinical detection, please refer to the sections on
The use of this drug may impair the mental and physical abilities required for driving a car or operating heavy machinery.
Potentiation of the effects of alcohol may occur with the use of this drug.
Since there is no adequate experience in children who have received this drug, safety and efficacy in children have not been established.
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
Fluphenazine Hydrochloride Injection, USP may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
The safety for the use of this drug during pregnancy has not been established; therefore, the possible hazards should be weighed against the potential benefits when administering this drug to pregnant patients.
Fluphenazine Hydrochloride Injection, USP is indicated in the management of manifestations of psychotic disorders.
Fluphenazine hydrochloride has not been shown effective in the management of behavioral complications in patients with mental retardation.
The average well tolerated starting dose for adult psychotic patients is 1.25 mg (0.5 mL) intramuscularly. Depending on the severity and duration of symptoms, initial total daily dosage may range from 2.5 to 10 mg and should be divided and given at six to eight hour intervals.
The smallest amount that will produce the desired results must be carefully determined for each individual, since optimal dosage levels of this potent drug vary from patient to patient. In general, the parenteral dose for fluphenazine has been found to be approximately 1/3 to 1/2 the oral dose. Treatment may be instituted with a
When symptoms are controlled, oral maintenance therapy can generally be instituted often with single daily doses. Continued treatment by the oral route, if possible, is needed to achieve maximum therapeutic benefits; further adjustments in dosage may be necessary during the course of therapy to meet the patient’s requirements.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Phenothiazines are contraindicated in patients with suspected or established subcortical brain damage, in patients receiving large doses of hypnotics and in comatose or severely depressed states. The presence of blood dyscrasia or liver damage precludes the use of fluphenazine hydrochloride. Fluphenazine hydrochloride is contraindicated in patients who have shown hypersensitivity to fluphenazine; cross-sensitivity to phenothiazine derivatives may occur.
The side effects most frequently reported with phenothiazine compounds are extrapyramidal symptoms including pseudoparkinsonism, dystonia, dyskinesia, akathisia, oculogyric crises, opisthotonos and hyperreflexia. Most often these extrapyramidal symptoms are reversible; however, they may be persistent (see below). With any given phenothiazine derivative, the incidence and severity of such reactions depend more on individual patient sensitivity than on other factors, but dosage level and patient age are also determinants.
Extrapyramidal reactions may be alarming, and the patient should be forewarned and reassured. These reactions can usually be controlled by administration of antiparkinsonian drugs such as Benztropine Mesylate or Intravenous Caffeine and Sodium Benzoate Injection, and by subsequent reductions in dosage.
Fluphenazine Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of fluphenazine hydrochloride in Water for Injection, for intramuscular use for the management of schizophrenia. Fluphenazine hydrochloride is a trifluoromethyl phenothiazine derivative and the chemical name is 1-Piperazineethanol, 4-[3-[2-(trifluoromethyl)-10H-phenothiazin-10-yl] propyl]-,dihydrochloride and has the following structural formula:
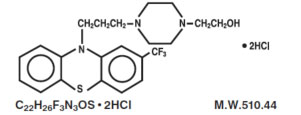
Each mL contains: Fluphenazine hydrochloride 2.5 mg; sodium chloride 9 mg to render the solution isotonic; methylparaben 1 mg and propylparaben 0.1 mg as preservatives; Water for Injection q.s. Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment (4.8 to 5.2).