Flurbiprofen Sodium
Flurbiprofen Sodium Prescribing Information
Flurbiprofen sodium ophthalmic solution is indicated for the inhibition of intraoperative miosis.
A total of four (4) drops of flurbiprofen sodium ophthalmic solution should be administered by instilling one (1) drop approximately every 1/2 hour beginning 2 hours before surgery.
Flurbiprofen sodium ophthalmic solution is contraindicated in individuals who are hypersensitive to any components of the medication.
Transient burning and stinging upon instillation and other minor symptoms of ocular irritation have been reported with the use of flurbiprofen sodium ophthalmic solution. Other adverse reactions reported with the use of flurbiprofen sodium ophthalmic solution include: fibrosis, hyphema, miosis, mydriasis, and ocular hyperemia.
Increased bleeding tendency of ocular tissues in conjunction with ocular surgery has also been reported
With some nonsteroidal anti-inflammatory drugs, there exists the potential for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that flurbiprofen sodium ophthalmic solution may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
There is the potential for cross-sensitivity to acetylsalicylic acid and other nonsteroidal anti-inflammatory drugs. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
Interaction of flurbiprofen sodium ophthalmic solution with other topical ophthalmic medications has not been fully investigated.
Although clinical studies with acetylcholine chloride and animal studies with acetylcholine chloride or carbachol revealed no interference, and there is no known pharmacological basis for an interaction, there have been reports that acetylcholine chloride and carbachol have been ineffective when used in patients treated with flurbiprofen sodium ophthalmic solution.
Flurbiprofen sodium ophthalmic solution, USP 0.03% is a sterile topical nonsteroidal anti-inflammatory product for ophthalmic use.
Sodium (±)-2-(2-fluoro-4-biphenylyl)propionate dihydrate.
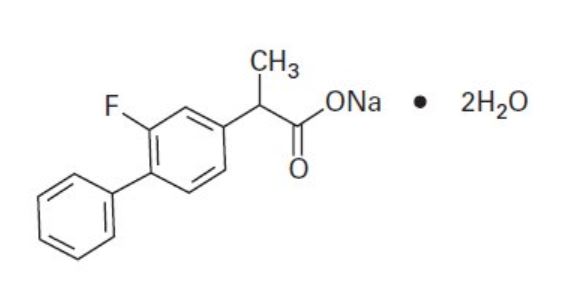
C15H12 FNaO2 •2H2O Mol. Wt. 302.27
Active: flurbiprofen sodium 0.03%.
Inactives: citric acid, edetate disodium, polyvinyl alcohol 1.4%, potassium chloride, purified water, sodium chloride, sodium citrate. hydrochloric acid and/or sodium hydroxide may be added to adjust pH (6.0 – 7.0).
Preservative: thimerosal 0.005%.