Focinvez
(Fosaprepitant Dimeglumine)Focinvez Prescribing Information
FOCINVEZ, in combination with other antiemetic agents, is indicated in adults and pediatric patients 6 months of age and older for the prevention of:
- acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high-dose cisplatin.
- delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC).
- FOCINVEZ has not been studied for the treatment of established nausea and vomiting.
Injection: 150 mg/50 mL (3 mg/mL) of fosaprepitant, a clear and colorless solution, in a single-dose vial.
FOCINVEZ is contraindicated in patients:
- who are hypersensitive to any component of the product. Hypersensitivity reactions including anaphylactic reactions, flushing, erythema, and dyspnea have been reported[see Warnings and Precautions.), and Adverse Reactions (
5.2 Hypersensitivity ReactionsSerious hypersensitivity reactions, including anaphylaxis and anaphylactic shock, during or soon after infusion of fosaprepitant have occurred. Symptoms including flushing, erythema, dyspnea, hypotension and syncope have been reported
[see Adverse Reactions ].Monitor patients during and after infusion. If hypersensitivity reactions occur, discontinue the infusion and administer appropriate medical therapy. Do not reinitiate FOCINVEZ in patients who experience these symptoms with previous use
[see Contraindications ].)]6.2 Postmarketing ExperienceThe following adverse reactions have been identified during post-approval use of fosaprepitant. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissue disorders: pruritus, rash, urticaria, Stevens-Johnson syndrome/toxic epidermal necrolysis[see Warnings and Precautions ].Immune system disorders:hypersensitivity reactions including anaphylaxis and anaphylactic shock[see Contraindications , Warnings and Precautions ].Nervous system disorders:ifosfamide-induced neurotoxicity reported after fosaprepitant and ifosfamide coadministration. - taking pimozide. Inhibition of CYP3A4 by aprepitant, the active moiety, could result in elevated plasma concentrations of this drug, which is a CYP3A4 substrate, potentially causing serious or life-threatening reactions, such as QT prolongation, a known adverse reaction of pimozide[see Warnings and Precautions (.)]
5.1 Clinically Significant CYP3A4 Drug InteractionsFosaprepitant, a prodrug of aprepitant, is a weak inhibitor of CYP3A4, and aprepitant is a substrate, inhibitor, and inducer of CYP3A4.
- Use of FOCINVEZ with other drugs that are CYP3A4 substrates, may result in increased plasma concentration of the concomitant drug.
- Use of pimozide with FOCINVEZ is contraindicated due to the risk of significantly increased plasma concentrations of pimozide, potentially resulting in prolongation of the QT interval, a known adverse reaction of pimozide[see Contraindications (4)].
- Use of FOCINVEZ with strong or moderate CYP3A4 inhibitors (e.g., ketoconazole, diltiazem) may increase plasma concentrations of aprepitant and result in an increased risk of adverse reactions related to FOCINVEZ.
- Use of FOCINVEZ with strong CYP3A4 inducers (e.g., rifampin) may result in a reduction in aprepitant plasma concentrations and decreased efficacy of FOCINVEZ.
See Table 7 and Table 8 for a listing of potentially significant drug interactions
[see Drug Interactions ].
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions[see Warnings and Precautions ()]
5.2 Hypersensitivity ReactionsSerious hypersensitivity reactions, including anaphylaxis and anaphylactic shock, during or soon after infusion of fosaprepitant have occurred. Symptoms including flushing, erythema, dyspnea, hypotension and syncope have been reported
[see Adverse Reactions ].Monitor patients during and after infusion. If hypersensitivity reactions occur, discontinue the infusion and administer appropriate medical therapy. Do not reinitiate FOCINVEZ in patients who experience these symptoms with previous use
[see Contraindications ]. - Infusion Site Reactions[see Warnings and Precautions ()]
5.3 Infusion Site ReactionsInfusion site reactions (ISRs) have been reported with the use of intravenous fosaprepitant
[see Adverse Reactions ]. The majority of severe ISRs, including thrombophlebitis and vasculitis, were reported with concomitant vesicant (anthracycline-based) chemotherapy administration, particularly when associated with extravasation. Necrosis was also reported in some patients with concomitant vesicant chemotherapy. Most ISRs occurred with the first, second or third exposure to single doses of intravenous fosaprepitant and in some cases, reactions persisted for two weeks or longer. Treatment of severe ISRs consisted of medical, and in some cases surgical, intervention.Avoid infusion of FOCINVEZ into small veins or through a butterfly catheter. If a severe ISR develops during infusion, discontinue the infusion and administer appropriate medical treatment.
FOCINVEZ (fosaprepitant injection) is a sterile, ready-to-use, clear and colorless solution formulation containing fosaprepitant dimeglumine, a prodrug of aprepitant, a substance P/neurokinin-1 (NK
1) receptor antagonist, an antiemetic agent, chemically described as 1-Deoxy-1-(methylamino)-D-glucitol[3-[[(2R,3S)-2-[(1R)-1-[3,5bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-2,5-dihydro-5-oxo-1
Its empirical formula is C
23H
22F
7N
4O
6P ⋅ 2(C
7H
17NO
5) and its structural formula is:
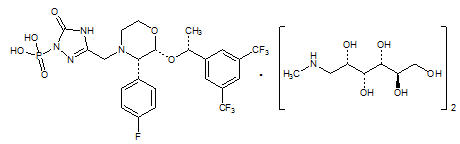
Fosaprepitant dimeglumine is a white to off-white amorphous powder with a molecular weight of 1004.83. It is freely soluble in water.
Each 50 mL vial of FOCINVEZ for administration as an intravenous infusion contains 150 mg of fosaprepitant (equivalent to 245.3 mg of fosaprepitant dimeglumine) and the following inactive ingredients: Betadex sulfobutyl ether sodium (8 g), edetate disodium (5.4 mg), sodium hydroxide (for pH adjustment) in water for injection.
The safety and efficacy of FOCINVEZ have been established based on adequate and well-controlled adult studies of another intravenous formulation of fosaprepitant for the prevention and of chemotherapy induced nausea and vomiting. Below is a description of the results of these adequate and well-controlled studies of fosaprepitant.