Fomepizole
Fomepizole Prescribing Information
Fomepizole injection is indicated as an antidote for ethylene glycol (such as antifreeze) or methanol poisoning, or for use in suspected ethylene glycol or methanol ingestion, either alone or in combination with hemodialysis (see
Treatment consists of blocking the formation of toxic metabolites using inhibitors of alcohol dehydrogenase, such as fomepizole injection, and correction of metabolic abnormalities. In patients with high ethylene glycol or methanol concentrations (≥ 50 mg/dL), significant metabolic acidosis, or renal failure, hemodialysis should be considered to remove ethylene glycol or methanol and the respective toxic metabolites of these alcohols.
A loading dose of 15 mg/kg should be administered, followed by doses of 10 mg/kg every 12 hours for 4 doses, then 15 mg/kg every 12 hours thereafter until ethylene glycol or methanol concentrations are undetectable or have been reduced below 20 mg/dL, and the patient is asymptomatic with normal pH. All doses should be administered as a slow intravenous infusion over 30 minutes (see
| DOSE AT THE BEGINNING OF HEMODIALYSIS | |
| DOSING DURING HEMODIALYSIS | |
| If <6 hours since last fomepizole injection dose | If ≥6 hours since last fomepizole injection dose |
| Do not administer dose | Administer next scheduled dose |
| Dose every 4 hours | |
| DOSING AT THE TIME HEMODIALYSIS IS COMPLETED | |
| Time between last dose and the end of hemodialysis | |
| <1 hour | Do not administer dose at the end of hemodialysis |
| 1–3 hours | Administer 1/2 of next scheduled dose |
| >3 hours | Administer next scheduled dose |
| MAINTENANCE DOSING OFF HEMODIALYSIS |
| Give next scheduled dose 12 hours from last dose administered |
Treatment consists of blocking the formation of toxic metabolites using inhibitors of alcohol dehydrogenase, such as fomepizole injection, and correction of metabolic abnormalities. In patients with high ethylene glycol or methanol concentrations (≥ 50 mg/dL), significant metabolic acidosis, or renal failure, hemodialysis should be considered to remove ethylene glycol or methanol and the respective toxic metabolites of these alcohols.
A loading dose of 15 mg/kg should be administered, followed by doses of 10 mg/kg every 12 hours for 4 doses, then 15 mg/kg every 12 hours thereafter until ethylene glycol or methanol concentrations are undetectable or have been reduced below 20 mg/dL, and the patient is asymptomatic with normal pH. All doses should be administered as a slow intravenous infusion over 30 minutes (see
| DOSE AT THE BEGINNING OF HEMODIALYSIS | |
| DOSING DURING HEMODIALYSIS | |
| If <6 hours since last fomepizole injection dose | If ≥6 hours since last fomepizole injection dose |
| Do not administer dose | Administer next scheduled dose |
| Dose every 4 hours | |
| DOSING AT THE TIME HEMODIALYSIS IS COMPLETED | |
| Time between last dose and the end of hemodialysis | |
| <1 hour | Do not administer dose at the end of hemodialysis |
| 1–3 hours | Administer 1/2 of next scheduled dose |
| >3 hours | Administer next scheduled dose |
| MAINTENANCE DOSING OFF HEMODIALYSIS |
| Give next scheduled dose 12 hours from last dose administered |
Fomepizole injection should not be administered to patients with a documented serious hypersensitivity reaction to fomepizole injection or other pyrazoles.
The most frequent adverse events reported as drug-related or unknown relationship to study drug in the 78 patients and 63 normal volunteers who received fomepizole injection were headache (14%), nausea (11%), and dizziness, increased drowsiness, and bad taste/metallic taste (6% each). All other adverse events in this population were reported in approximately 3% or fewer of those receiving fomepizole and were as follows:
Body as a Whole: Abdominal pain, fever, multiorgan system failure, pain during fomepizole injection, inflammation at injection site, lumbalgia/backache, hangover
Cardiovascular: Sinus bradycardia/bradycardia, phlebosclerosis, tachycardia, phlebitis, shock, hypotension
Gastrointestinal: Vomiting, diarrhea, dyspepsia, heartburn, decreased appetite, transient transaminitis
Hemic/Lymphatic: Eosinophilia/hypereosinophilia, lymphangitis, disseminated intravascular coagulation, anemia
Nervous: Lightheadedness, seizure, agitation, feeling drunk, facial flush, vertigo, nystagmus, anxiety, “felt strange”, decreased environmental awareness
Respiratory: Hiccups, pharyngitis
Skin/Appendages: Application site reaction, rash
Special Senses: Abnormal smell, speech/visual disturbances, transient blurred vision, roar in ear
Urogenital: Anuria
Drug Interactions
Oral doses of fomepizole (10-20 mg/kg), via alcohol dehydrogenase inhibition, significantly reduced the rate of elimination of ethanol (by approximately 40%) given to healthy volunteers in moderate doses. Similarly, ethanol decreased the rate of elimination of fomepizole (by approximately 50%) by the same mechanism.
Reciprocal interactions may occur with concomitant use of fomepizole and drugs that increase or inhibit the cytochrome P450 system (e.g., phenytoin, carbamazepine, cimetidine, ketoconazole), though this has not been studied.
Fomepizole injection is a competitive inhibitor of alcohol dehydrogenase.
The chemical name of fomepizole is 4-methylpyrazole. It has the molecular formula C4H6N2 and a molecular weight of 82.1. The structural formula is:
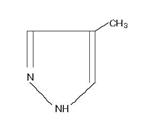
It is a clear to yellow liquid at room temperature. Its melting point is 25° C (77° F) and it may present in a solid form at room temperature. Fomepizole is soluble in water and very soluble in ethanol, diethyl ether, and chloroform. Each vial contains 1.5 mL (1 g/mL) of fomepizole.