Formoterol Fumarate
Formoterol Fumarate Prescribing Information
The recommended dose of formoterol fumarate inhalation solution is one 20 mcg unit-dose vial administered twice daily (morning and evening) by nebulization. A total daily dose greater than 40 mcg is not recommended.
Formoterol fumarate inhalation solution should be administered by the orally inhaled route via a standard jet nebulizer connected to an air compressor. The safety and efficacy of formoterol fumarate inhalation solution have been established in clinical trials when administered using the PARI-LC Plus
®nebulizer (with a facemask or mouthpiece) and the PRONEB
®Ultra compressor. The safety and efficacy of formoterol fumarate inhalation solution delivered from non-compressor based nebulizer systems have not been established.
Formoterol fumarate inhalation solution should always be stored in the foil pouch, and only removed IMMEDIATELY BEFORE USE. Contents of any partially used container should be discarded.
If the recommended maintenance treatment regimen fails to provide the usual response, medical advice should be sought immediately, as this is often a sign of destabilization of COPD. Under these circumstances, the therapeutic regimen should be re-evaluated and additional therapeutic options should be considered.
The drug compatibility (physical and chemical), efficacy, and safety of formoterol fumarate inhalation solution when mixed with other drugs in a nebulizer have not been established.
Formoterol fumarate inhalation solution is supplied as a sterile solution for nebulization in low-density polyethylene unit-dose vials. Each vial contains formoterol fumarate dihydrate, USP equivalent to 20 mcg/2 mL of formoterol fumarate.
Use of a long-acting beta
2-adrenergic agonists (LABA), including formoterol fumarate inhalation solution, without an inhaled corticosteroid is contraindicated in patients with asthma
- The safety and efficacy of formoterol fumarate inhalation solution in patients with asthma have not been established. Formoterol fumarate inhalation solution is not indicated for the treatment of asthma.[see CONTRAINDICATIONS ( 4)]
- Use of LABA as monotherapy [without inhaled corticosteroids (ICS)] for asthma is associated with an increased risk of asthma-related death. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone.
- A 28-week, placebo-controlled US study comparing the safety of another LABA (salmeterol) with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in patients receiving salmeterol (13/13,176 in patients treated with salmeterol vs. 3/13,179 in patients treated with placebo; RR 4.37, 95% CI 1.25, 15.34). The increased risk of asthma-related death is considered a class effect of the long-acting beta2-adrenergic agonists, including formoterol fumarate inhalation solution.
- No study adequate to determine whether the rate of asthma related death is increased in patients treated with formoterol fumarate inhalation solution has been conducted. Clinical studies with formoterol fumarate administered as a dry powder inhaler suggested a higher incidence of serious asthma exacerbations in patients who received formoterol than in those who received placebo. The sizes of these studies were not adequate to precisely quantify the differences in serious asthma exacerbation rates between treatment groups.
- Available data do not suggest an increased risk of death with use of LABA in patients with COPD.
Long-acting beta
2-adrenergic agonists, such as formoterol fumarate inhalation solution, as monotherapy (without an inhaled corticosteroid) for asthma increase the risk of asthma-related events. Formoterol fumarate inhalation solution is not indicated for the treatment of asthma
- The safety and efficacy of formoterol fumarate inhalation solution in patients with asthma have not been established. Formoterol fumarate inhalation solution is not indicated for the treatment of asthma.[see CONTRAINDICATIONS ( 4)]
- Use of LABA as monotherapy [without inhaled corticosteroids (ICS)] for asthma is associated with an increased risk of asthma-related death. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone.
- A 28-week, placebo-controlled US study comparing the safety of another LABA (salmeterol) with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in patients receiving salmeterol (13/13,176 in patients treated with salmeterol vs. 3/13,179 in patients treated with placebo; RR 4.37, 95% CI 1.25, 15.34). The increased risk of asthma-related death is considered a class effect of the long-acting beta2-adrenergic agonists, including formoterol fumarate inhalation solution.
- No study adequate to determine whether the rate of asthma related death is increased in patients treated with formoterol fumarate inhalation solution has been conducted. Clinical studies with formoterol fumarate administered as a dry powder inhaler suggested a higher incidence of serious asthma exacerbations in patients who received formoterol than in those who received placebo. The sizes of these studies were not adequate to precisely quantify the differences in serious asthma exacerbation rates between treatment groups.
- Available data do not suggest an increased risk of death with use of LABA in patients with COPD.
Formoterol fumarate inhalation solution is supplied as 2 mL of formoterol fumarate inhalation solution packaged in a 2.9 mL single-dose low-density polyethylene vial and overwrapped in a foil pouch. Each vial contains 2 mL of a clear, colorless solution composed of formoterol fumarate dihydrate, USP equivalent to 20 mcg of formoterol fumarate in an isotonic, sterile aqueous solution containing sodium chloride, water, pH adjusted to 5.0 with citric acid and sodium citrate.
The active component of formoterol fumarate inhalation solution is formoterol fumarate dihydrate, USP, a racemate. Formoterol fumarate dihydrate, USP is a beta
2-adrenergic bronchodilator. Its chemical name is (±)-2´-Hydroxy-5´-[(
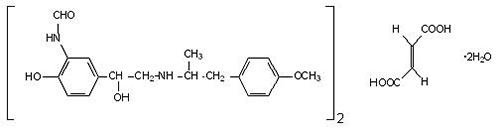
Formoterol fumarate dihydrate, USP has a molecular weight of 840.91 and its molecular formula is (C
19H
24N
2O
4)
2•C
4H
4O
4•2H
2O. Formoterol fumarate dihydrate, USP is a white to yellowish crystalline powder, which is freely soluble in glacial acetic acid, soluble in methanol, sparingly soluble in ethanol and isopropanol, slightly soluble in water, and practically insoluble in acetone, ethyl acetate, and diethyl ether.
Formoterol fumarate inhalation solution does not require dilution prior to administration by nebulization. Like all other nebulized treatments, the amount delivered to the lungs will depend on patient factors and the nebulization system used and its performance.
Using the PARI-LC Plus
® nebulizer (with a facemask or mouthpiece) connected to a PRONEB
® Ultra compressor under
Formoterol fumarate is a long-acting, beta
2-adrenergic receptor agonist (beta
2-agonist). Inhaled formoterol fumarate acts locally in the lung as a bronchodilator.
2-receptors than at beta
1-receptors. Although beta
2-receptors are the predominant adrenergic receptors in bronchial smooth muscle and beta
1-receptors are the predominant receptors in the heart, there are also beta
2-receptors in the human heart comprising 10% to 50% of the total beta-adrenergic receptors. The precise function of these receptors has not been established, but they raise the possibility that even highly selective beta
2-agonists may have cardiac effects.
The pharmacologic effects of beta
2-adrenoceptor agonist drugs, including formoterol, are at least in part attributable to stimulation of intracellular adenylyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3', 5'-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.