Foscarnet Prescribing Information
Foscarnet Sodium Injection is administered by controlled intravenous infusion, either by using a central venous line or by using a peripheral vein. The rate of infusion must be no more than 1 mg/kg/minute. An individualized dose of Foscarnet Sodium Injection should be calculated on the basis of body weight (mg/kg), renal function, indication of use and dosing frequency (refer to DOSAGE subsection).
An individualized dose at the required concentration (24 mg per mL or 12 mg per mL) for the route of administration (central line or peripheral line) needs to be aseptically prepared prior to dispensing. The standard 24 mg per mL solution may be used with or without dilution when using a central venous catheter for infusion. When a peripheral vein catheter is used, the 24 mg per mL injection
Hydration may reduce the risk of nephrotoxicity. Clinically dehydrated patients should have their condition corrected before initiating Foscarnet Sodium Injection therapy. It is recommended that 750–1000 mL of normal saline or 5% dextrose solution should be given prior to the first infusion of Foscarnet Sodium Injection to establish diuresis. With subsequent infusions, 750–1000 mL of hydration fluid should be given with 90–120 mg/kg of Foscarnet Sodium Injection, and 500 mL with 40–60 mg/kg of Foscarnet Sodium Injection. Hydration fluid may need to be decreased if clinically warranted. Oral rehydration with similar regimens may be considered in certain patients.
After the first dose, the hydration fluid should be administered concurrently with each infusion of Foscarnet Sodium Injection.
Other drugs and supplements can be administered to a patient receiving Foscarnet Sodium Injection. However, care must be taken to ensure that Foscarnet Sodium Injection is only administered with normal saline or 5% dextrose solution and that no other drug or supplement is administered concurrently via the same catheter. Foscarnet has been reported to be chemically incompatible with 30% dextrose, amphotericin B, and solutions containing calcium such as Ringer's lactate and TPN. Physical incompatibility with other IV drugs has also been reported including acyclovir sodium, ganciclovir, trimetrexate glucuronate, pentamidine isethionate, vancomycin, trimethoprim/sulfamethoxazole, diazepam, midazolam, digoxin, phenytoin, leucovorin, and proclorperazine. Because of foscarnet's chelating properties, a precipitate can potentially occur when divalent cations are administered concurrently in the same catheter.
Parenteral drug products must be inspected visually for particulate matter and discoloration prior to administration whenever the solution and container permit. Solutions that are discolored or contain particulate matter should not be used.
Accidental skin and eye contact with foscarnet sodium solution may cause local irritation and burning sensation. If accidental contact occurs, the exposed area should be flushed with water.
Foscarnet Sodium Injection is indicated for the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS). Combination therapy with Foscarnet Sodium Injection and ganciclovir is indicated for patients who have relapsed after monotherapy with either drug. SAFETY AND EFFICACY OF FOSCARNET SODIUM INJECTION HAVE NOT BEEN ESTABLISHED FOR TREATMENT OF OTHER CMV INFECTIONS (e.g., PNEUMONITIS, GASTROENTERITIS); CONGENITAL OR NEONATAL CMV DISEASE; OR NONIMMUNOCOMPROMISED INDIVIDUALS.
Foscarnet Sodium Injection is indicated for the treatment of acyclovir-resistant mucocutaneous HSV infections in immunocompromised patients. SAFETY AND EFFICACY OF FOSCARNET SODIUM INJECTION HAVE NOT BEEN ESTABLISHED FOR TREATMENT OF OTHER HSV INFECTIONS (e.g., RETINITIS, ENCEPHALITIS); CONGENITAL OR NEONATAL HSV DISEASE; OR HSV IN NONIMMUNOCOMPROMISED INDIVIDUALS.
Foscarnet Sodium Injection is contraindicated in patients with clinically significant hypersensitivity to foscarnet sodium.
THE MAJOR TOXICITY OF FOSCARNET IS RENAL IMPAIRMENT (see
THE MAJOR TOXICITY OF FOSCARNET IS RENAL IMPAIRMENT (see ADVERSE REACTIONSsection). Renal impairment is most likely to become clinically evident during the second week of induction therapy, but may occur at any time during foscarnet treatment. Renal function should be monitored carefully during both induction and maintenance therapy (see PATIENT MONITORINGsection). Elevations in serum creatinine are usually, but not always, reversible following discontinuation or dose adjustment of foscarnet. Safety and efficacy data for patients with baseline serum creatinine levels greater than 2.8 mg/dL or measured 24-hour creatinine clearances <50 mL/min are limited.
SINCE FOSCARNET HAS THE POTENTIAL TO CAUSE RENAL IMPAIRMENT, DOSE ADJUSTMENT BASED ON SERUM CREATININE IS NECESSARY. Hydration may reduce the risk of nephrotoxicity. It is recommended that 750–1000 mL of normal saline or 5% dextrose solution should be given prior to the first infusion of foscarnet to establish diuresis. With subsequent infusions, 750–1000 mL of hydration fluid should be given with 90-120 mg/kg of foscarnet, and 500 mL with 40–60 mg/kg of foscarnet. Hydration fluid may need to be decreased if clinically warranted.
After the first dose, the hydration fluid should be administered concurrently with each infusion of foscarnet.
Foscarnet has been associated with changes in serum electrolytes including hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, and hypokalemia (see ADVERSE REACTIONSsection). Foscarnet may also be associated with a dose-related decrease in ionized serum calcium which may not be reflected in total serum calcium. This effect is likely to be related to chelation of divalent metal ions such as calcium by foscarnet. Patients should be advised to report symptoms of low ionized calcium such as perioral tingling, numbness in the extremities and paresthesias. Particular caution and careful management of serum electrolytes is advised in patients with altered calcium or other electrolyte levels before treatment and especially in those with neurologic or cardiac abnormalities and those receiving other drugs known to influence minerals and electrolytes (see PATIENT MONITORINGand Drug Interactionssections). Physicians should be prepared to treat these abnormalities and their sequelae such as tetany, seizures or cardiac disturbances. The rate of foscarnet infusion may also affect the decrease in ionized calcium.
Seizures related to mineral and electrolyte abnormalities have been associated with foscarnet treatment (see WARNING section; Mineral And Electrolyte Abnormalities). Several cases of seizures were associated with death. Cases of status epilepticus have been reported. Risk factors associated with seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions.
Serious acute hypersensitivity reactions (e.g., anaphylactic shock, urticaria, angioedema) have been reported postmarketing in patients receiving foscarnet (see ADVERSE REACTIONSsection). If such an acute reaction occurs, therapy should be discontinued and appropriate medical therapy immediately instituted.
Foscarnet has been associated with prolongation of the QT interval, an ECG abnormality that has been associated with torsades de pointes, which has been reported during postmarketing surveillance for foscarnet (see ADVERSE REACTIONSsection). Some of these patients had confounding risk factors such as underlying cardiac disease, electrolyte abnormalities and other concomitant medications.
Use with caution in patients who have a history of QT prolongation, in patients who are taking medications known to prolong the QT interval (see PRECAUTIONSsection), in patients with electrolyte disturbances, or in patients who have other risk factors for QT prolongation. Electrocardiograms (ECGs) and measurement of electrolytes should be obtained prior to treatment initiation and periodically during treatment with foscarnet.
Foscarnet has been associated with changes in serum electrolytes including hypocalcemia (15-30%), hypophosphatemia (8–26%) and hyperphosphatemia (6%), hypomagnesemia (15–30%), and hypokalemia (16–48%) (see
THE MAJOR TOXICITY OF FOSCARNET IS RENAL IMPAIRMENT (see ADVERSE REACTIONSsection). Renal impairment is most likely to become clinically evident during the second week of induction therapy, but may occur at any time during foscarnet treatment. Renal function should be monitored carefully during both induction and maintenance therapy (see PATIENT MONITORINGsection). Elevations in serum creatinine are usually, but not always, reversible following discontinuation or dose adjustment of foscarnet. Safety and efficacy data for patients with baseline serum creatinine levels greater than 2.8 mg/dL or measured 24-hour creatinine clearances <50 mL/min are limited.
SINCE FOSCARNET HAS THE POTENTIAL TO CAUSE RENAL IMPAIRMENT, DOSE ADJUSTMENT BASED ON SERUM CREATININE IS NECESSARY. Hydration may reduce the risk of nephrotoxicity. It is recommended that 750–1000 mL of normal saline or 5% dextrose solution should be given prior to the first infusion of foscarnet to establish diuresis. With subsequent infusions, 750–1000 mL of hydration fluid should be given with 90-120 mg/kg of foscarnet, and 500 mL with 40–60 mg/kg of foscarnet. Hydration fluid may need to be decreased if clinically warranted.
After the first dose, the hydration fluid should be administered concurrently with each infusion of foscarnet.
Foscarnet has been associated with changes in serum electrolytes including hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, and hypokalemia (see ADVERSE REACTIONSsection). Foscarnet may also be associated with a dose-related decrease in ionized serum calcium which may not be reflected in total serum calcium. This effect is likely to be related to chelation of divalent metal ions such as calcium by foscarnet. Patients should be advised to report symptoms of low ionized calcium such as perioral tingling, numbness in the extremities and paresthesias. Particular caution and careful management of serum electrolytes is advised in patients with altered calcium or other electrolyte levels before treatment and especially in those with neurologic or cardiac abnormalities and those receiving other drugs known to influence minerals and electrolytes (see PATIENT MONITORINGand Drug Interactionssections). Physicians should be prepared to treat these abnormalities and their sequelae such as tetany, seizures or cardiac disturbances. The rate of foscarnet infusion may also affect the decrease in ionized calcium.
Seizures related to mineral and electrolyte abnormalities have been associated with foscarnet treatment (see WARNING section; Mineral And Electrolyte Abnormalities). Several cases of seizures were associated with death. Cases of status epilepticus have been reported. Risk factors associated with seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions.
Serious acute hypersensitivity reactions (e.g., anaphylactic shock, urticaria, angioedema) have been reported postmarketing in patients receiving foscarnet (see ADVERSE REACTIONSsection). If such an acute reaction occurs, therapy should be discontinued and appropriate medical therapy immediately instituted.
Foscarnet has been associated with prolongation of the QT interval, an ECG abnormality that has been associated with torsades de pointes, which has been reported during postmarketing surveillance for foscarnet (see ADVERSE REACTIONSsection). Some of these patients had confounding risk factors such as underlying cardiac disease, electrolyte abnormalities and other concomitant medications.
Use with caution in patients who have a history of QT prolongation, in patients who are taking medications known to prolong the QT interval (see PRECAUTIONSsection), in patients with electrolyte disturbances, or in patients who have other risk factors for QT prolongation. Electrocardiograms (ECGs) and measurement of electrolytes should be obtained prior to treatment initiation and periodically during treatment with foscarnet.
Foscarnet treatment was associated with seizures in 18/189 (10%) AIDS patients in the initial five controlled studies (see
THE MAJOR TOXICITY OF FOSCARNET IS RENAL IMPAIRMENT (see ADVERSE REACTIONSsection). Renal impairment is most likely to become clinically evident during the second week of induction therapy, but may occur at any time during foscarnet treatment. Renal function should be monitored carefully during both induction and maintenance therapy (see PATIENT MONITORINGsection). Elevations in serum creatinine are usually, but not always, reversible following discontinuation or dose adjustment of foscarnet. Safety and efficacy data for patients with baseline serum creatinine levels greater than 2.8 mg/dL or measured 24-hour creatinine clearances <50 mL/min are limited.
SINCE FOSCARNET HAS THE POTENTIAL TO CAUSE RENAL IMPAIRMENT, DOSE ADJUSTMENT BASED ON SERUM CREATININE IS NECESSARY. Hydration may reduce the risk of nephrotoxicity. It is recommended that 750–1000 mL of normal saline or 5% dextrose solution should be given prior to the first infusion of foscarnet to establish diuresis. With subsequent infusions, 750–1000 mL of hydration fluid should be given with 90-120 mg/kg of foscarnet, and 500 mL with 40–60 mg/kg of foscarnet. Hydration fluid may need to be decreased if clinically warranted.
After the first dose, the hydration fluid should be administered concurrently with each infusion of foscarnet.
Foscarnet has been associated with changes in serum electrolytes including hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, and hypokalemia (see ADVERSE REACTIONSsection). Foscarnet may also be associated with a dose-related decrease in ionized serum calcium which may not be reflected in total serum calcium. This effect is likely to be related to chelation of divalent metal ions such as calcium by foscarnet. Patients should be advised to report symptoms of low ionized calcium such as perioral tingling, numbness in the extremities and paresthesias. Particular caution and careful management of serum electrolytes is advised in patients with altered calcium or other electrolyte levels before treatment and especially in those with neurologic or cardiac abnormalities and those receiving other drugs known to influence minerals and electrolytes (see PATIENT MONITORINGand Drug Interactionssections). Physicians should be prepared to treat these abnormalities and their sequelae such as tetany, seizures or cardiac disturbances. The rate of foscarnet infusion may also affect the decrease in ionized calcium.
Seizures related to mineral and electrolyte abnormalities have been associated with foscarnet treatment (see WARNING section; Mineral And Electrolyte Abnormalities). Several cases of seizures were associated with death. Cases of status epilepticus have been reported. Risk factors associated with seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions.
Serious acute hypersensitivity reactions (e.g., anaphylactic shock, urticaria, angioedema) have been reported postmarketing in patients receiving foscarnet (see ADVERSE REACTIONSsection). If such an acute reaction occurs, therapy should be discontinued and appropriate medical therapy immediately instituted.
Foscarnet has been associated with prolongation of the QT interval, an ECG abnormality that has been associated with torsades de pointes, which has been reported during postmarketing surveillance for foscarnet (see ADVERSE REACTIONSsection). Some of these patients had confounding risk factors such as underlying cardiac disease, electrolyte abnormalities and other concomitant medications.
Use with caution in patients who have a history of QT prolongation, in patients who are taking medications known to prolong the QT interval (see PRECAUTIONSsection), in patients with electrolyte disturbances, or in patients who have other risk factors for QT prolongation. Electrocardiograms (ECGs) and measurement of electrolytes should be obtained prior to treatment initiation and periodically during treatment with foscarnet.
In controlled clinical trials performed in the United States, overdosage with foscarnet was reported in 10 out of 189 patients. All 10 patients experienced adverse events and all except one made a complete recovery. One patient died after receiving a total daily dose of 12.5 g for three days instead of the intended 10.9 g. The patient suffered a grand mal seizure and became comatose. Three days later the patient expired with the cause of death listed as respiratory/cardiac arrest. The other nine patients received doses ranging from 1.14 times to 8 times their recommended doses with an average of 4 times their recommended doses. Overall, three patients had seizures, three patients had renal function impairment, four patients had paresthesias either in limbs or periorally, and five patients had documented electrolyte disturbances primarily involving calcium and phosphate.
Overdose (up to 20 times the recommended dose) has been reported in post-marketing use of foscarnet. Some of these post-marketing reports were relative overdoses in that the dose of foscarnet had not been adjusted in patients with a reduced renal function. The pattern of adverse events associated with a foscarnet overdose is consistent with the known adverse event profile of the drug.
There is no specific antidote for foscarnet overdose. Hemodialysis and hydration may be of benefit in reducing drug plasma levels in patients who receive an overdosage of foscarnet, but the effectiveness of these interventions has not been evaluated. The patient should be observed for signs and symptoms of renal impairment and electrolyte imbalance. Medical treatment should be instituted if clinically warranted.
In five controlled U.S. clinical trials the most frequently reported adverse events in patients with AIDS and CMV retinitis are shown in
n = 189 | n = 189 | ||
Fever | 65% | Abnormal Renal Function | 27% |
Nausea | 47% | Vomiting | 26% |
Anemia | 33% | Headache | 26% |
Diarrhea | 30% | Seizures | 10% |
n = 189 | n = 189 | ||
Fever | 65% | Abnormal Renal Function | 27% |
Nausea | 47% | Vomiting | 26% |
Anemia | 33% | Headache | 26% |
Diarrhea | 30% | Seizures | 10% |
From the same controlled studies, adverse events categorized by investigator as “severe” are shown in
n = 189 | |
Death | 14% |
Abnormal Renal Function | 14% |
Marrow Suppression | 10% |
Anemia | 9% |
Seizures | 7% |
THE MAJOR TOXICITY OF FOSCARNET IS RENAL IMPAIRMENT (see ADVERSE REACTIONSsection). Renal impairment is most likely to become clinically evident during the second week of induction therapy, but may occur at any time during foscarnet treatment. Renal function should be monitored carefully during both induction and maintenance therapy (see PATIENT MONITORINGsection). Elevations in serum creatinine are usually, but not always, reversible following discontinuation or dose adjustment of foscarnet. Safety and efficacy data for patients with baseline serum creatinine levels greater than 2.8 mg/dL or measured 24-hour creatinine clearances <50 mL/min are limited.
SINCE FOSCARNET HAS THE POTENTIAL TO CAUSE RENAL IMPAIRMENT, DOSE ADJUSTMENT BASED ON SERUM CREATININE IS NECESSARY. Hydration may reduce the risk of nephrotoxicity. It is recommended that 750–1000 mL of normal saline or 5% dextrose solution should be given prior to the first infusion of foscarnet to establish diuresis. With subsequent infusions, 750–1000 mL of hydration fluid should be given with 90-120 mg/kg of foscarnet, and 500 mL with 40–60 mg/kg of foscarnet. Hydration fluid may need to be decreased if clinically warranted.
After the first dose, the hydration fluid should be administered concurrently with each infusion of foscarnet.
Foscarnet has been associated with changes in serum electrolytes including hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, and hypokalemia (see ADVERSE REACTIONSsection). Foscarnet may also be associated with a dose-related decrease in ionized serum calcium which may not be reflected in total serum calcium. This effect is likely to be related to chelation of divalent metal ions such as calcium by foscarnet. Patients should be advised to report symptoms of low ionized calcium such as perioral tingling, numbness in the extremities and paresthesias. Particular caution and careful management of serum electrolytes is advised in patients with altered calcium or other electrolyte levels before treatment and especially in those with neurologic or cardiac abnormalities and those receiving other drugs known to influence minerals and electrolytes (see PATIENT MONITORINGand Drug Interactionssections). Physicians should be prepared to treat these abnormalities and their sequelae such as tetany, seizures or cardiac disturbances. The rate of foscarnet infusion may also affect the decrease in ionized calcium.
Seizures related to mineral and electrolyte abnormalities have been associated with foscarnet treatment (see WARNING section; Mineral And Electrolyte Abnormalities). Several cases of seizures were associated with death. Cases of status epilepticus have been reported. Risk factors associated with seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions.
Serious acute hypersensitivity reactions (e.g., anaphylactic shock, urticaria, angioedema) have been reported postmarketing in patients receiving foscarnet (see ADVERSE REACTIONSsection). If such an acute reaction occurs, therapy should be discontinued and appropriate medical therapy immediately instituted.
Foscarnet has been associated with prolongation of the QT interval, an ECG abnormality that has been associated with torsades de pointes, which has been reported during postmarketing surveillance for foscarnet (see ADVERSE REACTIONSsection). Some of these patients had confounding risk factors such as underlying cardiac disease, electrolyte abnormalities and other concomitant medications.
Use with caution in patients who have a history of QT prolongation, in patients who are taking medications known to prolong the QT interval (see PRECAUTIONSsection), in patients with electrolyte disturbances, or in patients who have other risk factors for QT prolongation. Electrocardiograms (ECGs) and measurement of electrolytes should be obtained prior to treatment initiation and periodically during treatment with foscarnet.
n = 189 | |
Death | 14% |
Abnormal Renal Function | 14% |
Marrow Suppression | 10% |
Anemia | 9% |
Seizures | 7% |
From the five initial U.S. controlled trials of foscarnet, the following list of adverse events has been compiled regardless of causal relationship to foscarnet. Evaluation of these reports was difficult because of the diverse manifestations of the underlying disease and because most patients received numerous concomitant medications.
THE MAJOR TOXICITY OF FOSCARNET IS RENAL IMPAIRMENT (see ADVERSE REACTIONSsection). Renal impairment is most likely to become clinically evident during the second week of induction therapy, but may occur at any time during foscarnet treatment. Renal function should be monitored carefully during both induction and maintenance therapy (see PATIENT MONITORINGsection). Elevations in serum creatinine are usually, but not always, reversible following discontinuation or dose adjustment of foscarnet. Safety and efficacy data for patients with baseline serum creatinine levels greater than 2.8 mg/dL or measured 24-hour creatinine clearances <50 mL/min are limited.
SINCE FOSCARNET HAS THE POTENTIAL TO CAUSE RENAL IMPAIRMENT, DOSE ADJUSTMENT BASED ON SERUM CREATININE IS NECESSARY. Hydration may reduce the risk of nephrotoxicity. It is recommended that 750–1000 mL of normal saline or 5% dextrose solution should be given prior to the first infusion of foscarnet to establish diuresis. With subsequent infusions, 750–1000 mL of hydration fluid should be given with 90-120 mg/kg of foscarnet, and 500 mL with 40–60 mg/kg of foscarnet. Hydration fluid may need to be decreased if clinically warranted.
After the first dose, the hydration fluid should be administered concurrently with each infusion of foscarnet.
Foscarnet has been associated with changes in serum electrolytes including hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, and hypokalemia (see ADVERSE REACTIONSsection). Foscarnet may also be associated with a dose-related decrease in ionized serum calcium which may not be reflected in total serum calcium. This effect is likely to be related to chelation of divalent metal ions such as calcium by foscarnet. Patients should be advised to report symptoms of low ionized calcium such as perioral tingling, numbness in the extremities and paresthesias. Particular caution and careful management of serum electrolytes is advised in patients with altered calcium or other electrolyte levels before treatment and especially in those with neurologic or cardiac abnormalities and those receiving other drugs known to influence minerals and electrolytes (see PATIENT MONITORINGand Drug Interactionssections). Physicians should be prepared to treat these abnormalities and their sequelae such as tetany, seizures or cardiac disturbances. The rate of foscarnet infusion may also affect the decrease in ionized calcium.
Seizures related to mineral and electrolyte abnormalities have been associated with foscarnet treatment (see WARNING section; Mineral And Electrolyte Abnormalities). Several cases of seizures were associated with death. Cases of status epilepticus have been reported. Risk factors associated with seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions.
Serious acute hypersensitivity reactions (e.g., anaphylactic shock, urticaria, angioedema) have been reported postmarketing in patients receiving foscarnet (see ADVERSE REACTIONSsection). If such an acute reaction occurs, therapy should be discontinued and appropriate medical therapy immediately instituted.
Foscarnet has been associated with prolongation of the QT interval, an ECG abnormality that has been associated with torsades de pointes, which has been reported during postmarketing surveillance for foscarnet (see ADVERSE REACTIONSsection). Some of these patients had confounding risk factors such as underlying cardiac disease, electrolyte abnormalities and other concomitant medications.
Use with caution in patients who have a history of QT prolongation, in patients who are taking medications known to prolong the QT interval (see PRECAUTIONSsection), in patients with electrolyte disturbances, or in patients who have other risk factors for QT prolongation. Electrocardiograms (ECGs) and measurement of electrolytes should be obtained prior to treatment initiation and periodically during treatment with foscarnet.
Care must be taken to infuse solutions containing foscarnet only into veins with adequate blood flow to permit rapid dilution and distribution to avoid local irritation (see DOSAGE AND ADMINISTRATION). Local irritation and ulcerations of penile epithelium have been reported in male patients receiving foscarnet, possibly related to the presence of drug in the urine. Cases of male and female genital irritation/ulceration have been reported in patients receiving foscarnet. Adequate hydration with close attention to personal hygiene may minimize the occurrence of such events.
Due to the sodium content of foscarnet sodium injection (240 micromoles (5.5 mg) of sodium per mL), avoid foscarnet sodium injection use when intravenous infusion of a large amount of sodium or water may not be tolerated (e.g. in patients with cardiomyopathy). Foscarnet sodium injection should also be avoided in patients on a controlled sodium diet.
Anemia has been reported in 33% of patients receiving foscarnet in controlled studies. Granulocytopenia has been reported in 17% of patients receiving foscarnet in controlled studies; however, only 1% (2/189) were terminated from these studies because of neutropenia.
CMV Retinitis: Patients should be advised that foscarnet is not a cure for CMV retinitis, and that they may continue to experience progression of retinitis during or following treatment. They should be advised to have regular ophthalmologic examinations.
Because of foscarnet's tendency to cause renal impairment, the use of foscarnet should be avoided in combination with potentially nephrotoxic drugs such as aminoglycosides, amphotericin B, cyclosporine, acyclovir, methotrexate, tacrolimus and intravenous pentamidine (see above) unless the potential benefits outweigh the risks to the patient.
When diuretics are indicated, thiazides are recommended over loop diuretics because the latter inhibit renal tubular secretion, and may impair elimination of foscarnet, potentially leading to toxicity.
Abnormal renal function has been observed in clinical practice during the use of foscarnet and ritonavir, or foscarnet, ritonavir, and saquinavir. (See DOSAGE and ADMINISTRATION.)
Because of the risk of QT prolongation and the potential for torsades de pointes, the use of foscarnet should be avoided in combination with agents known to prolong the QT interval including Class IA (e.g., quinidine or procainamide) or Class III (e.g., dofetilide, amiodarone, sotalol) antiarrhythmic agents, phenothiazines, tricyclic antidepressants, and certain macrolides and fluoroquinolones.
Carcinogenicity studies were conducted in rats and mice at oral doses of 500 mg/kg/day and 250 mg/kg/day. Oral bioavailability in unfasted rodents is < 20%. No evidence of oncogenicity was reported at plasma drug levels equal to 1/3 and 1/5, respectively, of those in humans (at the maximum recommended human daily dose) as measured by the area-under-the-time/concentration curve (AUC).
Foscarnet showed genotoxic effects in the BALB/3T3
There are no adequate and well-controlled studies of foscarnet in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Animal Data: Foscarnet did not adversely affect fertility and general reproductive performance in rats. The results of peri- and post-natal studies in rats were also negative. However, these studies used exposures that are inadequate to define the potential for impairment of fertility at human drug exposure levels.
Daily subcutaneous doses up to 75 mg/kg administered to female rats prior to and during mating, during gestation, and 21 days post-partum caused a slight increase (< 5%) in the number of skeletal anomalies compared with the control group. Daily subcutaneous doses up to 75 mg/kg administered to rabbits and 150 mg/kg administered to rats during gestation caused an increase in the frequency of skeletal anomalies/variations. On the basis of estimated drug exposure (as measured by AUC), the 150 mg/kg dose in rats and 75 mg/kg dose in rabbits were approximately one-eighth (rat) and one-third (rabbit) the estimated maximal daily human exposure. These studies are inadequate to define the potential teratogenicity at levels to which women will be exposed.
It is not known whether foscarnet is excreted in human milk; however, in lactating rats administered 75 mg/kg, foscarnet was excreted in maternal milk at concentrations three times higher than peak maternal blood concentrations. Because of the potential for serious adverse events in nursing infants, a decision should be made whether to discontinue nursing or discontinue drug, taking into consideration the importance of the drug to the mother. The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV.
The safety and effectiveness of foscarnet in pediatric patients have not been established. Foscarnet is deposited in teeth and bone and deposition is greater in young and growing animals. Foscarnet has been demonstrated to adversely affect development of tooth enamel in mice and rats. The effects of this deposition on skeletal development have not been studied.
Since deposition in human bone has also been shown to occur, it is likely that it does so to a greater degree in developing bone in pediatric patients. Administration to pediatric patients should be undertaken only after careful evaluation and only if the potential benefits for treatment outweigh the risks.
No studies of the efficacy or safety of foscarnet in persons 65 years of age or older have been conducted. However, foscarnet has been used in patients age 65 years of age and older. The pattern of adverse events seen in these patients is consistent across all age groups. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and renal function should be monitored. (See DOSAGE AND ADMINISTRATION).
THE MAJOR TOXICITY OF FOSCARNET IS RENAL IMPAIRMENT (see ADVERSE REACTIONSsection). Renal impairment is most likely to become clinically evident during the second week of induction therapy, but may occur at any time during foscarnet treatment. Renal function should be monitored carefully during both induction and maintenance therapy (see PATIENT MONITORINGsection). Elevations in serum creatinine are usually, but not always, reversible following discontinuation or dose adjustment of foscarnet. Safety and efficacy data for patients with baseline serum creatinine levels greater than 2.8 mg/dL or measured 24-hour creatinine clearances <50 mL/min are limited.
SINCE FOSCARNET HAS THE POTENTIAL TO CAUSE RENAL IMPAIRMENT, DOSE ADJUSTMENT BASED ON SERUM CREATININE IS NECESSARY. Hydration may reduce the risk of nephrotoxicity. It is recommended that 750–1000 mL of normal saline or 5% dextrose solution should be given prior to the first infusion of foscarnet to establish diuresis. With subsequent infusions, 750–1000 mL of hydration fluid should be given with 90-120 mg/kg of foscarnet, and 500 mL with 40–60 mg/kg of foscarnet. Hydration fluid may need to be decreased if clinically warranted.
After the first dose, the hydration fluid should be administered concurrently with each infusion of foscarnet.
Foscarnet has been associated with changes in serum electrolytes including hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, and hypokalemia (see ADVERSE REACTIONSsection). Foscarnet may also be associated with a dose-related decrease in ionized serum calcium which may not be reflected in total serum calcium. This effect is likely to be related to chelation of divalent metal ions such as calcium by foscarnet. Patients should be advised to report symptoms of low ionized calcium such as perioral tingling, numbness in the extremities and paresthesias. Particular caution and careful management of serum electrolytes is advised in patients with altered calcium or other electrolyte levels before treatment and especially in those with neurologic or cardiac abnormalities and those receiving other drugs known to influence minerals and electrolytes (see PATIENT MONITORINGand Drug Interactionssections). Physicians should be prepared to treat these abnormalities and their sequelae such as tetany, seizures or cardiac disturbances. The rate of foscarnet infusion may also affect the decrease in ionized calcium.
Seizures related to mineral and electrolyte abnormalities have been associated with foscarnet treatment (see WARNING section; Mineral And Electrolyte Abnormalities). Several cases of seizures were associated with death. Cases of status epilepticus have been reported. Risk factors associated with seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions.
Serious acute hypersensitivity reactions (e.g., anaphylactic shock, urticaria, angioedema) have been reported postmarketing in patients receiving foscarnet (see ADVERSE REACTIONSsection). If such an acute reaction occurs, therapy should be discontinued and appropriate medical therapy immediately instituted.
Foscarnet has been associated with prolongation of the QT interval, an ECG abnormality that has been associated with torsades de pointes, which has been reported during postmarketing surveillance for foscarnet (see ADVERSE REACTIONSsection). Some of these patients had confounding risk factors such as underlying cardiac disease, electrolyte abnormalities and other concomitant medications.
Use with caution in patients who have a history of QT prolongation, in patients who are taking medications known to prolong the QT interval (see PRECAUTIONSsection), in patients with electrolyte disturbances, or in patients who have other risk factors for QT prolongation. Electrocardiograms (ECGs) and measurement of electrolytes should be obtained prior to treatment initiation and periodically during treatment with foscarnet.
THE MAJOR TOXICITY OF FOSCARNET IS RENAL IMPAIRMENT (see ADVERSE REACTIONSsection). Renal impairment is most likely to become clinically evident during the second week of induction therapy, but may occur at any time during foscarnet treatment. Renal function should be monitored carefully during both induction and maintenance therapy (see PATIENT MONITORINGsection). Elevations in serum creatinine are usually, but not always, reversible following discontinuation or dose adjustment of foscarnet. Safety and efficacy data for patients with baseline serum creatinine levels greater than 2.8 mg/dL or measured 24-hour creatinine clearances <50 mL/min are limited.
SINCE FOSCARNET HAS THE POTENTIAL TO CAUSE RENAL IMPAIRMENT, DOSE ADJUSTMENT BASED ON SERUM CREATININE IS NECESSARY. Hydration may reduce the risk of nephrotoxicity. It is recommended that 750–1000 mL of normal saline or 5% dextrose solution should be given prior to the first infusion of foscarnet to establish diuresis. With subsequent infusions, 750–1000 mL of hydration fluid should be given with 90-120 mg/kg of foscarnet, and 500 mL with 40–60 mg/kg of foscarnet. Hydration fluid may need to be decreased if clinically warranted.
After the first dose, the hydration fluid should be administered concurrently with each infusion of foscarnet.
Foscarnet has been associated with changes in serum electrolytes including hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, and hypokalemia (see ADVERSE REACTIONSsection). Foscarnet may also be associated with a dose-related decrease in ionized serum calcium which may not be reflected in total serum calcium. This effect is likely to be related to chelation of divalent metal ions such as calcium by foscarnet. Patients should be advised to report symptoms of low ionized calcium such as perioral tingling, numbness in the extremities and paresthesias. Particular caution and careful management of serum electrolytes is advised in patients with altered calcium or other electrolyte levels before treatment and especially in those with neurologic or cardiac abnormalities and those receiving other drugs known to influence minerals and electrolytes (see PATIENT MONITORINGand Drug Interactionssections). Physicians should be prepared to treat these abnormalities and their sequelae such as tetany, seizures or cardiac disturbances. The rate of foscarnet infusion may also affect the decrease in ionized calcium.
Seizures related to mineral and electrolyte abnormalities have been associated with foscarnet treatment (see WARNING section; Mineral And Electrolyte Abnormalities). Several cases of seizures were associated with death. Cases of status epilepticus have been reported. Risk factors associated with seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions.
Serious acute hypersensitivity reactions (e.g., anaphylactic shock, urticaria, angioedema) have been reported postmarketing in patients receiving foscarnet (see ADVERSE REACTIONSsection). If such an acute reaction occurs, therapy should be discontinued and appropriate medical therapy immediately instituted.
Foscarnet has been associated with prolongation of the QT interval, an ECG abnormality that has been associated with torsades de pointes, which has been reported during postmarketing surveillance for foscarnet (see ADVERSE REACTIONSsection). Some of these patients had confounding risk factors such as underlying cardiac disease, electrolyte abnormalities and other concomitant medications.
Use with caution in patients who have a history of QT prolongation, in patients who are taking medications known to prolong the QT interval (see PRECAUTIONSsection), in patients with electrolyte disturbances, or in patients who have other risk factors for QT prolongation. Electrocardiograms (ECGs) and measurement of electrolytes should be obtained prior to treatment initiation and periodically during treatment with foscarnet.
THE MAJOR TOXICITY OF FOSCARNET IS RENAL IMPAIRMENT (see ADVERSE REACTIONSsection). Renal impairment is most likely to become clinically evident during the second week of induction therapy, but may occur at any time during foscarnet treatment. Renal function should be monitored carefully during both induction and maintenance therapy (see PATIENT MONITORINGsection). Elevations in serum creatinine are usually, but not always, reversible following discontinuation or dose adjustment of foscarnet. Safety and efficacy data for patients with baseline serum creatinine levels greater than 2.8 mg/dL or measured 24-hour creatinine clearances <50 mL/min are limited.
SINCE FOSCARNET HAS THE POTENTIAL TO CAUSE RENAL IMPAIRMENT, DOSE ADJUSTMENT BASED ON SERUM CREATININE IS NECESSARY. Hydration may reduce the risk of nephrotoxicity. It is recommended that 750–1000 mL of normal saline or 5% dextrose solution should be given prior to the first infusion of foscarnet to establish diuresis. With subsequent infusions, 750–1000 mL of hydration fluid should be given with 90-120 mg/kg of foscarnet, and 500 mL with 40–60 mg/kg of foscarnet. Hydration fluid may need to be decreased if clinically warranted.
After the first dose, the hydration fluid should be administered concurrently with each infusion of foscarnet.
Foscarnet has been associated with changes in serum electrolytes including hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, and hypokalemia (see ADVERSE REACTIONSsection). Foscarnet may also be associated with a dose-related decrease in ionized serum calcium which may not be reflected in total serum calcium. This effect is likely to be related to chelation of divalent metal ions such as calcium by foscarnet. Patients should be advised to report symptoms of low ionized calcium such as perioral tingling, numbness in the extremities and paresthesias. Particular caution and careful management of serum electrolytes is advised in patients with altered calcium or other electrolyte levels before treatment and especially in those with neurologic or cardiac abnormalities and those receiving other drugs known to influence minerals and electrolytes (see PATIENT MONITORINGand Drug Interactionssections). Physicians should be prepared to treat these abnormalities and their sequelae such as tetany, seizures or cardiac disturbances. The rate of foscarnet infusion may also affect the decrease in ionized calcium.
Seizures related to mineral and electrolyte abnormalities have been associated with foscarnet treatment (see WARNING section; Mineral And Electrolyte Abnormalities). Several cases of seizures were associated with death. Cases of status epilepticus have been reported. Risk factors associated with seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions.
Serious acute hypersensitivity reactions (e.g., anaphylactic shock, urticaria, angioedema) have been reported postmarketing in patients receiving foscarnet (see ADVERSE REACTIONSsection). If such an acute reaction occurs, therapy should be discontinued and appropriate medical therapy immediately instituted.
Foscarnet has been associated with prolongation of the QT interval, an ECG abnormality that has been associated with torsades de pointes, which has been reported during postmarketing surveillance for foscarnet (see ADVERSE REACTIONSsection). Some of these patients had confounding risk factors such as underlying cardiac disease, electrolyte abnormalities and other concomitant medications.
Use with caution in patients who have a history of QT prolongation, in patients who are taking medications known to prolong the QT interval (see PRECAUTIONSsection), in patients with electrolyte disturbances, or in patients who have other risk factors for QT prolongation. Electrocardiograms (ECGs) and measurement of electrolytes should be obtained prior to treatment initiation and periodically during treatment with foscarnet.
THE MAJOR TOXICITY OF FOSCARNET IS RENAL IMPAIRMENT (see ADVERSE REACTIONSsection). Renal impairment is most likely to become clinically evident during the second week of induction therapy, but may occur at any time during foscarnet treatment. Renal function should be monitored carefully during both induction and maintenance therapy (see PATIENT MONITORINGsection). Elevations in serum creatinine are usually, but not always, reversible following discontinuation or dose adjustment of foscarnet. Safety and efficacy data for patients with baseline serum creatinine levels greater than 2.8 mg/dL or measured 24-hour creatinine clearances <50 mL/min are limited.
SINCE FOSCARNET HAS THE POTENTIAL TO CAUSE RENAL IMPAIRMENT, DOSE ADJUSTMENT BASED ON SERUM CREATININE IS NECESSARY. Hydration may reduce the risk of nephrotoxicity. It is recommended that 750–1000 mL of normal saline or 5% dextrose solution should be given prior to the first infusion of foscarnet to establish diuresis. With subsequent infusions, 750–1000 mL of hydration fluid should be given with 90-120 mg/kg of foscarnet, and 500 mL with 40–60 mg/kg of foscarnet. Hydration fluid may need to be decreased if clinically warranted.
After the first dose, the hydration fluid should be administered concurrently with each infusion of foscarnet.
Foscarnet has been associated with changes in serum electrolytes including hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, and hypokalemia (see ADVERSE REACTIONSsection). Foscarnet may also be associated with a dose-related decrease in ionized serum calcium which may not be reflected in total serum calcium. This effect is likely to be related to chelation of divalent metal ions such as calcium by foscarnet. Patients should be advised to report symptoms of low ionized calcium such as perioral tingling, numbness in the extremities and paresthesias. Particular caution and careful management of serum electrolytes is advised in patients with altered calcium or other electrolyte levels before treatment and especially in those with neurologic or cardiac abnormalities and those receiving other drugs known to influence minerals and electrolytes (see PATIENT MONITORINGand Drug Interactionssections). Physicians should be prepared to treat these abnormalities and their sequelae such as tetany, seizures or cardiac disturbances. The rate of foscarnet infusion may also affect the decrease in ionized calcium.
Seizures related to mineral and electrolyte abnormalities have been associated with foscarnet treatment (see WARNING section; Mineral And Electrolyte Abnormalities). Several cases of seizures were associated with death. Cases of status epilepticus have been reported. Risk factors associated with seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions.
Serious acute hypersensitivity reactions (e.g., anaphylactic shock, urticaria, angioedema) have been reported postmarketing in patients receiving foscarnet (see ADVERSE REACTIONSsection). If such an acute reaction occurs, therapy should be discontinued and appropriate medical therapy immediately instituted.
Foscarnet has been associated with prolongation of the QT interval, an ECG abnormality that has been associated with torsades de pointes, which has been reported during postmarketing surveillance for foscarnet (see ADVERSE REACTIONSsection). Some of these patients had confounding risk factors such as underlying cardiac disease, electrolyte abnormalities and other concomitant medications.
Use with caution in patients who have a history of QT prolongation, in patients who are taking medications known to prolong the QT interval (see PRECAUTIONSsection), in patients with electrolyte disturbances, or in patients who have other risk factors for QT prolongation. Electrocardiograms (ECGs) and measurement of electrolytes should be obtained prior to treatment initiation and periodically during treatment with foscarnet.
Selected adverse events occurring at a rate of less than 1% in the five initial U.S. controlled clinical trials of foscarnet include: syndrome of inappropriate antidiuretic hormone secretion, pancytopenia, hematuria, dehydration, hypoproteinemia, increases in amylase and creatinine phosphokinase, cardiac arrest, coma, and other cardiovascular and neurologic complications.
Selected adverse event data from the Foscarnet vs. Ganciclovir CMV Retinitis Trial (FGCRT), performed by the Studies of the Ocular Complications of AIDS (SOCA) Research Group, are shown in
* Values for the treatment groups refer only to patients who completed at least one follow-up visit – i.e., 133 to 119 patients in the ganciclovir group and 93 to 100 in the foscarnet group. “Events” denotes all events observed and “patients” the number of patients with one or more of the indicated events. | ||||||
†Per person-year at risk | ||||||
‡Final frozen SOCA I database dated October 1991 | ||||||
EVENT | GANCICLOVIR | FOSCARNET | ||||
| No. of Events | No. of Patients | Rates† | No. of Events | No. of Patients | Rates† | |
| Absolute neutrophil count decreasing to <0.50 x 109per liter | 63 | 41 | 1.30 | 31 | 17 | 0.72 |
| Serum creatinine increasing to >260 μmol per liter (>2.9 mg/dL) | 6 | 4 | 0.12 | 13 | 9 | 0.30 |
| Seizure ‡ | 21 | 13 | 0.37 | 19 | 13 | 0.37 |
| Catheterization-related infection | 49 | 27 | 1.26 | 51 | 28 | 1.46 |
| Hospitalization | 209 | 91 | 4.74 | 202 | 75 | 5.03 |
A prospective, randomized, controlled clinical trial (FOS-03) was conducted in 24 patients with AIDS and CMV retinitis comparing treatment with foscarnet to no treatment. Patients received induction treatment of foscarnet, 60 mg/kg every 8 hours for 3 weeks, followed by maintenance treatment with 90 mg/kg/day until retinitis progression (appearance of a new lesion or advancement of the border of a posterior lesion greater than 750 microns in diameter). All diagnoses and determinations of retinitis progression were made from masked reading of retinal photographs. The 13 patients randomized to treatment with foscarnet had a significant delay in progression of CMV retinitis compared to untreated controls. Median times to retinitis progression from study entry were 93 days (range 21 – >364) and 22 days (range 7 – 42), respectively.
In another prospective clinical trial of CMV retinitis in patients with AIDS (ACTG-915), 33 patients were treated with two to three weeks of foscarnet induction (60 mg/kg TID) and then randomized to either 90 mg/kg/day or 120 mg/kg/day maintenance therapy. The median times from study entry to retinitis progression were not significantly different between the treatment groups, 96 (range 14 – >176) days and 140 (range 16 – >233) days, respectively.
In study ACTG 129/FGCRT SOCA study 107 patients with newly diagnosed CMV retinitis were randomized to treatment with foscarnet (induction: 60 mg/kg TID for 2 weeks; maintenance: 90 mg/kg QD) and 127 were randomized to treatment with ganciclovir (induction: 5 mg/kg BID; maintenance: 5 mg/kg QD). The median time to progression on the two drugs was similar (Fos=59 and Gcv=56 days).
The CMV Retinitis Retreatment Trial (ACTG 228/SOCA CRRT) was a randomized, open-label comparison of foscarnet or ganciclovir monotherapy to the combination of both drugs for the treatment of persistently active or relapsed CMV retinitis in patients with AIDS. Subjects were randomized to one of the three treatments: foscarnet 90 mg/kg BID induction followed by 120 mg/kg QD maintenance (Fos); ganciclovir 5 mg/kg BID induction followed by 10 mg/kg QD maintenance (Gcv); or the combination of the two drugs, consisting of continuation of the subject's current therapy and induction dosing of the other drug (as above), followed by maintenance with foscarnet 90 mg/kg QD plus ganciclovir 5 mg/kg QD (Cmb). Assessment of retinitis progression was performed by masked evaluation of retinal photographs. The median times to retinitis progression or death were 39 days for the foscarnet group, 61 days for the ganciclovir group and 105 days for the combination group. For the alternative endpoint of retinitis progression (censoring on death), the median times were 39 days for the foscarnet group, 61 days for the ganciclovir group and 132 days for the combination group. Due to censoring on death, the latter analysis may overestimate the treatment effect. Treatment modifications due to toxicity were more common in the combination group than in the foscarnet or ganciclovir monotherapy groups (see ADVERSE REACTIONSsection).
In a controlled trial, patients with AIDS and mucocutaneous, acyclovir-resistant HSV infection were randomized to either foscarnet (N=8) at a dose of 40 mg/kg TID or vidarabine (N=6) at a dose of 15 mg/kg per day. Eleven patients were nonrandomly assigned to receive treatment with foscarnet because of prior intolerance to vidarabine. Lesions in the eight patients randomized to foscarnet healed after 11 to 25 days; seven of the 11 patients nonrandomly treated with foscarnet healed their lesions in 10 to 30 days. Vidarabine was discontinued because of intolerance (N=4) or poor therapeutic response (N=2). In a second trial, forty AIDS patients and three bone marrow transplant recipients with mucocutaneous, acyclovir-resistant HSV infections were randomized to receive foscarnet at a dose of either 40 mg/kg BID or 40 mg/kg TID. Fifteen of the 43 patients had healing of their lesions in 11 to 72 days with no difference in response between the two treatment groups.
* Values for the treatment groups refer only to patients who completed at least one follow-up visit – i.e., 133 to 119 patients in the ganciclovir group and 93 to 100 in the foscarnet group. “Events” denotes all events observed and “patients” the number of patients with one or more of the indicated events. | ||||||
†Per person-year at risk | ||||||
‡Final frozen SOCA I database dated October 1991 | ||||||
EVENT | GANCICLOVIR | FOSCARNET | ||||
| No. of Events | No. of Patients | Rates† | No. of Events | No. of Patients | Rates† | |
| Absolute neutrophil count decreasing to <0.50 x 109 per liter | 63 | 41 | 1.30 | 31 | 17 | 0.72 |
| Serum creatinine increasing to >260 μmol per liter (>2.9 mg/dL) | 6 | 4 | 0.12 | 13 | 9 | 0.30 |
| Seizure ‡ | 21 | 13 | 0.37 | 19 | 13 | 0.37 |
| Catheterization-related infection | 49 | 27 | 1.26 | 51 | 28 | 1.46 |
| Hospitalization | 209 | 91 | 4.74 | 202 | 75 | 5.03 |
Selected adverse events from ACTG Study 228 (CRRT) comparing combination therapy with foscarnet or ganciclovir monotherapy are shown in
* Pts. = patients with event; †Rate = events/person/year; ‡ANC = absolute neutrophil count | |||||||||
| Foscarnet N=88 | Ganciclovir N=93 | Combination N=93 | |||||||
| No. Events | No. Pts.* | Rate† | No. Events | No. Pts.* | Rate† | No. Events | No. Pts.* | Rate† | |
| Anemia (Hgb <70g/L) | 11 | 7 | 0.20 | 9 | 7 | 0.14 | 19 | 15 | 0.33 |
| Neutropenia‡ ANC <0.75 x 109cells/L ANC <0.50 x 109cells/L | 86 50 | 32 25 | 1.53 0.91 | 95 49 | 41 28 | 1.51 0.80 | 107 50 | 51 28 | 1.91 0.85 |
| Thrombocytopenia Platelets <50 x 109/L Platelets <20 x 109/L | 28 1 | 14 1 | 0.50 0.01 | 19 6 | 8 2 | 0.43 0.05 | 40 7 | 15 6 | 0.56 0.18 |
| Nephrotoxicity Creatinine >260 μmol/L (>2.9 mg/dL) | 9 | 7 | 0.15 | 10 | 7 | 0.17 | 11 | 10 | 0.20 |
| Seizures | 6 | 6 | 0.17 | 7 | 6 | 0.15 | 10 | 5 | 0.18 |
| Hospitalizations | 86 | 53 | 1.86 | 111 | 59 | 2.36 | 118 | 64 | 2.36 |
* Pts. = patients with event; †Rate = events/person/year; ‡ANC = absolute neutrophil count | |||||||||
| Foscarnet N=88 | Ganciclovir N=93 | Combination N=93 | |||||||
| No. Events | No. Pts.* | Rate† | No. Events | No. Pts.* | Rate† | No. Events | No. Pts.* | Rate† | |
| Anemia (Hgb <70g/L) | 11 | 7 | 0.20 | 9 | 7 | 0.14 | 19 | 15 | 0.33 |
| Neutropenia‡ ANC <0.75 x 109 cells/L ANC <0.50 x 109 cells/L | 86 50 | 32 25 | 1.53 0.91 | 95 49 | 41 28 | 1.51 0.80 | 107 50 | 51 28 | 1.91 0.85 |
| Thrombocytopenia Platelets <50 x 109/L Platelets <20 x 109/L | 28 1 | 14 1 | 0.50 0.01 | 19 6 | 8 2 | 0.43 0.05 | 40 7 | 15 6 | 0.56 0.18 |
| Nephrotoxicity Creatinine >260 μmol/L (>2.9 mg/dL) | 9 | 7 | 0.15 | 10 | 7 | 0.17 | 11 | 10 | 0.20 |
| Seizures | 6 | 6 | 0.17 | 7 | 6 | 0.15 | 10 | 5 | 0.18 |
| Hospitalizations | 86 | 53 | 1.86 | 111 | 59 | 2.36 | 118 | 64 | 2.36 |
Adverse events that have been reported in post-marketing surveillance include: administration site extravasation, localized edema, hypersensitivity reactions (including anaphylactic shock, urticaria and angioedema) (see
THE MAJOR TOXICITY OF FOSCARNET IS RENAL IMPAIRMENT (see ADVERSE REACTIONSsection). Renal impairment is most likely to become clinically evident during the second week of induction therapy, but may occur at any time during foscarnet treatment. Renal function should be monitored carefully during both induction and maintenance therapy (see PATIENT MONITORINGsection). Elevations in serum creatinine are usually, but not always, reversible following discontinuation or dose adjustment of foscarnet. Safety and efficacy data for patients with baseline serum creatinine levels greater than 2.8 mg/dL or measured 24-hour creatinine clearances <50 mL/min are limited.
SINCE FOSCARNET HAS THE POTENTIAL TO CAUSE RENAL IMPAIRMENT, DOSE ADJUSTMENT BASED ON SERUM CREATININE IS NECESSARY. Hydration may reduce the risk of nephrotoxicity. It is recommended that 750–1000 mL of normal saline or 5% dextrose solution should be given prior to the first infusion of foscarnet to establish diuresis. With subsequent infusions, 750–1000 mL of hydration fluid should be given with 90-120 mg/kg of foscarnet, and 500 mL with 40–60 mg/kg of foscarnet. Hydration fluid may need to be decreased if clinically warranted.
After the first dose, the hydration fluid should be administered concurrently with each infusion of foscarnet.
Foscarnet has been associated with changes in serum electrolytes including hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, and hypokalemia (see ADVERSE REACTIONSsection). Foscarnet may also be associated with a dose-related decrease in ionized serum calcium which may not be reflected in total serum calcium. This effect is likely to be related to chelation of divalent metal ions such as calcium by foscarnet. Patients should be advised to report symptoms of low ionized calcium such as perioral tingling, numbness in the extremities and paresthesias. Particular caution and careful management of serum electrolytes is advised in patients with altered calcium or other electrolyte levels before treatment and especially in those with neurologic or cardiac abnormalities and those receiving other drugs known to influence minerals and electrolytes (see PATIENT MONITORINGand Drug Interactionssections). Physicians should be prepared to treat these abnormalities and their sequelae such as tetany, seizures or cardiac disturbances. The rate of foscarnet infusion may also affect the decrease in ionized calcium.
Seizures related to mineral and electrolyte abnormalities have been associated with foscarnet treatment (see WARNING section; Mineral And Electrolyte Abnormalities). Several cases of seizures were associated with death. Cases of status epilepticus have been reported. Risk factors associated with seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions.
Serious acute hypersensitivity reactions (e.g., anaphylactic shock, urticaria, angioedema) have been reported postmarketing in patients receiving foscarnet (see ADVERSE REACTIONSsection). If such an acute reaction occurs, therapy should be discontinued and appropriate medical therapy immediately instituted.
Foscarnet has been associated with prolongation of the QT interval, an ECG abnormality that has been associated with torsades de pointes, which has been reported during postmarketing surveillance for foscarnet (see ADVERSE REACTIONSsection). Some of these patients had confounding risk factors such as underlying cardiac disease, electrolyte abnormalities and other concomitant medications.
Use with caution in patients who have a history of QT prolongation, in patients who are taking medications known to prolong the QT interval (see PRECAUTIONSsection), in patients with electrolyte disturbances, or in patients who have other risk factors for QT prolongation. Electrocardiograms (ECGs) and measurement of electrolytes should be obtained prior to treatment initiation and periodically during treatment with foscarnet.
THE MAJOR TOXICITY OF FOSCARNET IS RENAL IMPAIRMENT (see ADVERSE REACTIONSsection). Renal impairment is most likely to become clinically evident during the second week of induction therapy, but may occur at any time during foscarnet treatment. Renal function should be monitored carefully during both induction and maintenance therapy (see PATIENT MONITORINGsection). Elevations in serum creatinine are usually, but not always, reversible following discontinuation or dose adjustment of foscarnet. Safety and efficacy data for patients with baseline serum creatinine levels greater than 2.8 mg/dL or measured 24-hour creatinine clearances <50 mL/min are limited.
SINCE FOSCARNET HAS THE POTENTIAL TO CAUSE RENAL IMPAIRMENT, DOSE ADJUSTMENT BASED ON SERUM CREATININE IS NECESSARY. Hydration may reduce the risk of nephrotoxicity. It is recommended that 750–1000 mL of normal saline or 5% dextrose solution should be given prior to the first infusion of foscarnet to establish diuresis. With subsequent infusions, 750–1000 mL of hydration fluid should be given with 90-120 mg/kg of foscarnet, and 500 mL with 40–60 mg/kg of foscarnet. Hydration fluid may need to be decreased if clinically warranted.
After the first dose, the hydration fluid should be administered concurrently with each infusion of foscarnet.
Foscarnet has been associated with changes in serum electrolytes including hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, and hypokalemia (see ADVERSE REACTIONSsection). Foscarnet may also be associated with a dose-related decrease in ionized serum calcium which may not be reflected in total serum calcium. This effect is likely to be related to chelation of divalent metal ions such as calcium by foscarnet. Patients should be advised to report symptoms of low ionized calcium such as perioral tingling, numbness in the extremities and paresthesias. Particular caution and careful management of serum electrolytes is advised in patients with altered calcium or other electrolyte levels before treatment and especially in those with neurologic or cardiac abnormalities and those receiving other drugs known to influence minerals and electrolytes (see PATIENT MONITORINGand Drug Interactionssections). Physicians should be prepared to treat these abnormalities and their sequelae such as tetany, seizures or cardiac disturbances. The rate of foscarnet infusion may also affect the decrease in ionized calcium.
Seizures related to mineral and electrolyte abnormalities have been associated with foscarnet treatment (see WARNING section; Mineral And Electrolyte Abnormalities). Several cases of seizures were associated with death. Cases of status epilepticus have been reported. Risk factors associated with seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions.
Serious acute hypersensitivity reactions (e.g., anaphylactic shock, urticaria, angioedema) have been reported postmarketing in patients receiving foscarnet (see ADVERSE REACTIONSsection). If such an acute reaction occurs, therapy should be discontinued and appropriate medical therapy immediately instituted.
Foscarnet has been associated with prolongation of the QT interval, an ECG abnormality that has been associated with torsades de pointes, which has been reported during postmarketing surveillance for foscarnet (see ADVERSE REACTIONSsection). Some of these patients had confounding risk factors such as underlying cardiac disease, electrolyte abnormalities and other concomitant medications.
Use with caution in patients who have a history of QT prolongation, in patients who are taking medications known to prolong the QT interval (see PRECAUTIONSsection), in patients with electrolyte disturbances, or in patients who have other risk factors for QT prolongation. Electrocardiograms (ECGs) and measurement of electrolytes should be obtained prior to treatment initiation and periodically during treatment with foscarnet.
Because of foscarnet's tendency to cause renal impairment, the use of foscarnet should be avoided in combination with potentially nephrotoxic drugs such as aminoglycosides, amphotericin B, cyclosporine, acyclovir, methotrexate, tacrolimus and intravenous pentamidine (see above) unless the potential benefits outweigh the risks to the patient.
When diuretics are indicated, thiazides are recommended over loop diuretics because the latter inhibit renal tubular secretion, and may impair elimination of foscarnet, potentially leading to toxicity.
Abnormal renal function has been observed in clinical practice during the use of foscarnet and ritonavir, or foscarnet, ritonavir, and saquinavir. (See
Because of the risk of QT prolongation and the potential for torsades de pointes, the use of foscarnet should be avoided in combination with agents known to prolong the QT interval including Class IA (e.g., quinidine or procainamide) or Class III (e.g., dofetilide, amiodarone, sotalol) antiarrhythmic agents, phenothiazines, tricyclic antidepressants, and certain macrolides and fluoroquinolones.
Foscarnet Sodium Injection is the brand name for foscarnet sodium. The chemical name of foscarnet sodium is phosphonoformic acid, trisodium salt. Foscarnet sodium is a white to almost white crystalline powder containing 6 equivalents of water of hydration with an empirical formula of Na3CO5P•6 H2O and a molecular weight of 300.04. The structural formula is:
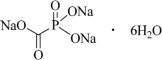
Foscarnet Sodium Injection has the potential to chelate divalent metal ions, such as calcium and magnesium, to form stable coordination compounds. Foscarnet Sodium Injection is a sterile, isotonic aqueous solution for intravenous administration only. The solution is clear and colorless. Each milliliter of Foscarnet Sodium Injection contains 24 mg of foscarnet sodium hexahydrate in Water for Injection, USP. Hydrochloric acid may have been added to adjust the pH of the solution to 7.4. Foscarnet Sodium Injection contains no preservatives.