Fosphenytoin Sodium - Fosphenytoin Sodium injection
(Fosphenytoin Sodium)Fosphenytoin Sodium - Fosphenytoin Sodium injection Prescribing Information
Fosphenytoin sodium injection, USP is indicated for the control of generalized tonic-clonic status epilepticus and prevention and treatment of seizures occurring during neurosurgery. Fosphenytoin can also be substituted, short-term, for oral phenytoin. Fosphenytoin should be used only when oral phenytoin administration is not possible. Fosphenytoin must not be given orally.
Caution must be used when administering fosphenytoin due to the risk of dosing errors (see
Prior to intravenous infusion, dilute fosphenytoin injection in 5% dextrose or 0.9% saline solution for injection to a concentration ranging from 1.5 to 25 mg PE/mL. The maximum concentration of fosphenytoin in any solution should be 25 mg PE/mL. When fosphenytoin is given as an intravenous infusion, fosphenytoin needs to be diluted and should only be administered at a rate not exceeding 150 mg PE/min.
Parenteral drug products should be inspected visually for particulate matter or discoloration prior to administration, whenever solution and container permit.
Status Epilepticus
- The loading dose of fosphenytoin is 15 to 20 mg PE/kg administered at 100 to 150 mg PE/min.
- Because of the risk of hypotension, fosphenytoin should be administered no faster than 150 mg PE/min.Continuous monitoring of the electrocardiogram, blood pressure, and respiratory function is essential and the patient should be observed throughout the period where maximal serum phenytoin concentrations occur, approximately 10 to 20 minutes after the end of fosphenytoin infusions.
- Because the full antiepileptic effect of phenytoin, whether given as fosphenytoin or parenteral phenytoin, is not immediate, other measures, including concomitant administration of an intravenous benzodiazepine, will usually be necessary for the control of status epilepticus.
- The loading dose should be followed by maintenance doses of either fosphenytoin or phenytoin.
If administration of fosphenytoin does not terminate seizures, the use of other anticonvulsants and other appropriate measures should be considered.
Even though loading doses of fosphenytoin have been given by the intramuscular route for other indications when intravenous access is impossible, intramuscular fosphenytoin should ordinarily not be used in the treatment of status epilepticus because therapeutic phenytoin concentrations may not be reached as quickly as with intravenous administration.
Nonemergent Loading and Maintenance Dosing
Because of the risks of cardiac and local toxicity associated with intravenous fosphenytoin, oral phenytoin should be used whenever possible.
The loading dose of fosphenytoin is 10 to 20 mg PE/kg given intravenous or intramuscular. The rate of administration for intravenous fosphenytoin should be no greater than 150 mg PE/min. Continuous monitoring of the electrocardiogram, blood pressure, and respiratory function is essential and the patient should be observed throughout the period where maximal serum phenytoin concentrations occur (approximately 20 minutes after the end of fosphenytoin infusion).
The initial daily maintenance dose of fosphenytoin is 4 to 6 mg PE/kg/day in divided doses.
When treatment with oral phenytoin is not possible, fosphenytoin can be substituted for oral phenytoin at the same total daily dose.
Phenytoin sodium capsules are approximately 90% bioavailable by the oral route. Phenytoin, supplied as fosphenytoin, is 100% bioavailable by both the intramuscular and intravenous routes. For this reason, plasma phenytoin concentrations may increase modestly when intramuscular or intravenous fosphenytoin is substituted for oral phenytoin sodium therapy.
The rate of administration for intravenous fosphenytoin should be no greater than 150 mg PE/min.
In controlled trials, intramuscular fosphenytoin was administered as a single daily dose utilizing either 1 or 2 injection sites. Some patients may require more frequent dosing.
Fosphenytoin sodium injection, USP is contraindicated in patients who have demonstrated hypersensitivity to fosphenytoin sodium injection, USP or its ingredients, or to phenytoin or other hydantoins.
Because of the effect of parenteral phenytoin on ventricular automaticity, fosphenytoin injection is contraindicated in patients with sinus bradycardia, sino-atrial block, second and third degree A-V block, and Adams-Stokes syndrome.
Coadministration of fosphenytoin is contraindicated with delavirdine due to potential for loss of virologic response and possible resistance to delavirdine or to the class of non-nucleoside reverse transcriptase inhibitors.
The more important adverse clinical events caused by the IV use of fosphenytoin or phenytoin are cardiovascular collapse and/or central nervous system depression. Hypotension can occur when either drug is administered rapidly by the IV route. The rate of administration is very important; for fosphenytoin, it should not exceed 150 mg PE/min.
The adverse clinical events most commonly observed with the use of fosphenytoin in clinical trials were nystagmus, dizziness, pruritus, paresthesia, headache, somnolence, and ataxia. With two exceptions, these events are commonly associated with the administration of IV phenytoin. Paresthesia and pruritus, however, were seen much more often following fosphenytoin administration and occurred more often with IV fosphenytoin administration than with IM fosphenytoin administration. These events were dose and rate related; most alert patients (41 of 64; 64%) administered doses of ≥15 mg PE/kg at 150 mg PE/min experienced discomfort of some degree. These sensations, generally described as itching, burning, or tingling, were usually not at the infusion site. The location of the discomfort varied with the groin mentioned most frequently as a site of involvement. The paresthesia and pruritus were transient events that occurred within several minutes of the start of infusion and generally resolved within 10 minutes after completion of fosphenytoin infusion. Some patients experienced symptoms for hours. These events did not increase in severity with repeated administration. Concurrent adverse events or clinical laboratory change suggesting an allergic process were not seen (see
Approximately 2% of the 859 individuals who received fosphenytoin in premarketing clinical trials discontinued treatment because of an adverse event. The adverse events most commonly associated with withdrawal were pruritus (0.5%), hypotension (0.3%), and bradycardia (0.2%).
The incidence of adverse events tended to increase as both dose and infusion rate increased. In particular, at doses of ≥15 mg PE/kg and rates ≥150 mg PE/min, transient pruritus, tinnitus, nystagmus, somnolence, and ataxia occurred 2 to 3 times more often than at lower doses or rates.
All adverse events were recorded during the trials by the clinical investigators using terminology of their own choosing. Similar types of events were grouped into standardized categories using modified COSTART dictionary terminology. These categories are used in the tables and listings below with the frequencies representing the proportion of individuals exposed to fosphenytoin or comparative therapy.
The prescriber should be aware that these figures cannot be used to predict the frequency of adverse events in the course of usual medical practice where patient characteristics and other factors may differ from those prevailing during clinical studies. Similarly, the cited frequencies cannot be directly compared with figures obtained from other clinical investigations involving different treatments, uses or investigators. An inspection of these frequencies, however, does provide the prescribing physician with one basis to estimate the relative contribution of drug and nondrug factors to the adverse event incidences in the population studied.
| TABLE 2. Treatment-Emergent Adverse Event Incidence Following IV Administration at the Maximum Dose and Rate to Patients with Epilepsy or Neurosurgical Patients (Events in at Least 2% of Fosphenytoin-Treated Patients) | ||
| BODY SYSTEM Adverse Event | IV Fosphenytoin N=90 | IV Phenytoin N=22 |
| BODY AS A WHOLE | ||
| Pelvic Pain | 4.4 | 0 |
| Asthenia | 2.2 | 0 |
| Back Pain | 2.2 | 0 |
| Headache | 2.2 | 4.5 |
| CARDIOVASCULAR | ||
| Hypotension | 7.7 | 9.1 |
| Vasodilatation | 5.6 | 4.5 |
| Tachycardia | 2.2 | 0 |
| DIGESTIVE | ||
| Nausea | 8.9 | 13.6 |
| Tongue Disorder | 4.4 | 0 |
| Dry Mouth | 4.4 | 4.5 |
| Vomiting | 2.2 | 9.1 |
| NERVOUS | ||
| Nystagmus | 44.4 | 59.1 |
| Dizziness | 31.1 | 27.3 |
| Somnolence | 20 | 27.3 |
| Ataxia | 11.1 | 18.2 |
| Stupor | 7.7 | 4.5 |
| Incoordination | 4.4 | 4.5 |
| Paresthesia | 4.4 | 0 |
| Extrapyramidal Syndrome | 4.4 | 0 |
| Tremor | 3.3 | 9.1 |
| Agitation | 3.3 | 0 |
| Hypesthesia | 2.2 | 9.1 |
| Dysarthria | 2.2 | 0 |
| Vertigo | 2.2 | 0 |
| Brain Edema | 2.2 | 4.5 |
| SKIN AND APPENDAGES | ||
| Pruritus | 48.9 | 4.5 |
| SPECIAL SENSES | ||
| Tinnitus | 8.9 | 9.1 |
| Diplopia | 3.3 | 0 |
| Taste Perversion | 3.3 | 0 |
| Amblyopia | 2.2 | 9.1 |
| Deafness | 2.2 | 0 |
| TABLE 3. Treatment-Emergent Adverse Event Incidence Following Substitution of IM Fosphenytoin for Oral Phenytoin Sodium in Patients With Epilepsy (Events in at Least 2% of Fosphenytoin-Treated Patients) | ||
| BODY SYSTEM Adverse Event | IM Fosphenytoin N=179 | Oral Phenytoin Sodium N=61 |
| BODY AS A WHOLE | ||
| Headache | 8.9 | 4.9 |
| Asthenia | 3.9 | 3.3 |
| Accidental Injury | 3.4 | 6.6 |
| DIGESTIVE | ||
| Nausea | 4.5 | 0 |
| Vomiting | 2.8 | 0 |
| HEMATOLOGIC AND LYMPHATIC | ||
| Ecchymosis | 7.3 | 4.9 |
| NERVOUS | ||
| Nystagmus | 15.1 | 8.2 |
| Tremor | 9.5 | 13.1 |
| Ataxia | 8.4 | 8.2 |
| Incoordination | 7.8 | 4.9 |
| Somnolence | 6.7 | 9.8 |
| Dizziness | 5 | 3.3 |
| Paresthesia | 3.9 | 3.3 |
| Reflexes Decreased | 2.8 | 4.9 |
| SKIN AND APPENDAGES | ||
| Pruritus | 2.8 | 0 |
Fosphenytoin has been administered to 859 individuals during all clinical trials. All adverse events seen at least twice are listed in the following, except those already included in previous tables and listings. Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in greater than 1/100 individuals; infrequent adverse events are those occurring in 1/100 to 1/1000 individuals.
hypochromic anemia, leukopenia, lymphadenopathy, petechia.
The following adverse reactions have been identified during postapproval use of fosphenytoin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
There have been post-marketing reports of anaphylactoid reaction and anaphylaxis.
Dyskinesia.
No drugs are known to interfere with the conversion of fosphenytoin to phenytoin. Conversion could be affected by alterations in the level of phosphatase activity, but given the abundance and wide distribution of phosphatases in the body it is unlikely that drugs would affect this activity enough to affect conversion of fosphenytoin to phenytoin. Drugs highly bound to albumin could increase the unbound fraction of fosphenytoin. Although, it is unknown whether this could result in clinically significant effects, caution is advised when administering fosphenytoin with other drugs that significantly bind to serum albumin.
The pharmacokinetics and protein binding of fosphenytoin, phenytoin, and diazepam were not altered when diazepam and fosphenytoin were concurrently administered in single submaximal doses.
The most significant drug interactions following administration of fosphenytoin are expected to occur with drugs that interact with phenytoin. Phenytoin is extensively bound to serum plasma proteins and is prone to competitive displacement. Phenytoin is metabolized by hepatic cytochrome P450 enzymes CYP2C9 and CYP2C19 and is particularly susceptible to inhibitory drug interactions because it is subject to saturable metabolism. Inhibition of metabolism may produce significant increases in circulating phenytoin concentrations and enhance the risk of drug toxicity. Phenytoin is a potent inducer of hepatic drug-metabolizing enzymes.
The most commonly occurring drug interactions are listed below:
Note: The list is not intended to be inclusive or comprehensive. Individual drug package inserts should be consulted.
- Drugs that may increase plasma phenytoin concentrations include: acute alcohol intake, amiodarone, anti-epileptic agents (ethosuximide, felbamate, oxcarbazepine, methsuximide, topiramate), azoles (fluconazole, ketoconazole, itraconazole, miconazole, voriconazole), capecitabine, chloramphenicol, chlordiazepoxide, disulfiram, estrogens, fluorouracil, fluoxetine, fluvastatin, fluvoxamine, H2-antagonists (e.g. cimetidine), halothane, isoniazid, methylphenidate, omeprazole, phenothiazines, salicylates, sertraline, succinimides, sulfonamides (e.g., sulfamethizole, sulfaphenazole, sulfadiazine, sulfamethoxazole-trimethoprim), tacrolimus, ticlopidine, tolbutamide, trazodone and warfarin.
- Drugs that may decrease plasma phenytoin concentrations include: anticancer drugs usually in combination (e.g., bleomycin, carboplatin, cisplatin, doxorubicin, methotrexate), carbamazepine, chronic alcohol abuse, diazepam, diazoxide, folic acid, fosamprenavir, nelfinavir, reserpine, rifampin, ritonavir, St. John’s Wort, theophylline and vigabatrin.
- Drugs that may either increase or decrease plasma phenytoin concentrations include: phenobarbital, valproic acid and sodium valproate. Similarly, the effects of phenytoin on phenobarbital, valproic acid and sodium plasma valproate concentrations are unpredictable.
- The addition or withdrawal of these agents in patients on phenytoin therapy may require an adjustment of the phenytoin dose to achieve optimal clinical outcome.
- Drugs that should not be coadministered with phenytoin: Delavirdine (see CONTRAINDICATIONS).
- Drugs whose efficacy is impaired by phenytoin include: azoles (fluconazole, ketoconazole, itraconazole, voriconazole, posaconazole), corticosteroids, doxycycline, estrogens, furosemide, irinotecan, oral contraceptives, paclitaxel, paroxetine, quinidine, rifampin, sertraline, teniposide, theophylline and vitamin D.
- Increased and decreased PT/INR responses have been reported when phenytoin is coadministered with warfarin.
- Phenytoin decreases plasma concentrations of active metabolites of albendazole, certain HIV antivirals (efavirenz, lopinavir/ritonavir, indinavir, nelfinavir, ritonavir, saquinavir), anti-epileptic agents (carbamazepine, felbamate, lamotrigine, topiramate, oxcarbazepine, quetiapine), atorvastatin, chlorpropamide, clozapine, cyclosporine, digoxin, fluvastatin, folic acid, methadone, mexiletine, nifedipine, nimodipine, nisoldipine, praziquantel, simvastatin and verapamil.
- Phenytoin when given with fosamprenavir alone may decrease the concentration of amprenavir, the active metabolite. Phenytoin when given with the combination of fosamprenavir and ritonavir may increase the concentration of amprenavir.
- Resistance to the neuromuscular blocking action of the nondepolarizing neuromuscular blocking agents pancuronium, vecuronium, rocuronium and cisatracurium has occurred in patients chronically administered phenytoin. Whether or not phenytoin has the same effect on other non-depolarizing agents is unknown. Patients should be monitored closely for more rapid recovery from neuromuscular blockade than expected, and infusion rate requirements may be higher.
- The addition or withdrawal of phenytoin during concomitant therapy with these agents may require adjustment of the dose of these agents to achieve optimal clinical outcome.
Monitoring of plasma phenytoin concentrations may be helpful when possible drug interactions are suspected (see
Phenytoin doses are usually selected to attain therapeutic plasma total phenytoin concentrations of 10 to 20 mcg/mL, (unbound phenytoin concentrations of 1 to 2 mcg/mL). Following fosphenytoin administration, it is recommended that phenytoin concentrations
Prior to complete conversion, commonly used immunoanalytical techniques, such as TDx®/TDxFLxTM(fluorescence polarization) and Emit®2000 (enzyme multiplied), may significantly overestimate plasma phenytoin concentrations because of cross-reactivity with fosphenytoin. The error is dependent on plasma phenytoin and fosphenytoin concentration (influenced by fosphenytoin dose, route and rate of administration, and time of sampling relative to dosing), and analytical method. Chromatographic assay methods accurately quantitate phenytoin concentrations in biological fluids in the presence of fosphenytoin. Prior to complete conversion, blood samples for phenytoin monitoring should be collected in tubes containing EDTA as an anticoagulant to minimize
Fosphenytoin sodium injection, USP is a prodrug intended for parenteral administration; its active metabolite is phenytoin. 1.5 mg fosphenytoin sodium, USP (hereafter referred to as fosphenytoin) equivalent to 1 mg phenytoin sodium and is referred to as 1 mg phenytoin equivalents (PE). The amount and concentration of fosphenytoin is always expressed in terms of mg PE.
Fosphenytoin injection is marketed in 2 mL vials containing a total of 100 mg PE and 10 mL vials containing a total of 500 mg PE. The concentration of each vial is 50 mg PE/mL. Fosphenytoin is supplied in vials as a ready-mixed solution in Water for Injection, USP, and Tromethamine, USP (TRIS), buffer adjusted to pH 8.6 to 9.0 with either Hydrochloric Acid, NF, or Sodium Hydroxide, NF. Fosphenytoin injection is a clear, colorless to pale yellow, sterile solution.
The chemical name of fosphenytoin is 5,5-diphenyl-3-[(phosphonooxy)methyl]-2,4-imidazolidinedione disodium salt.
The molecular structure of fosphenytoin is:
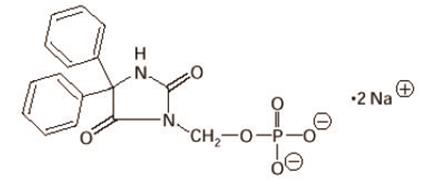
The molecular weight of fosphenytoin is 406.24.