Fulvestrant
Fulvestrant Prescribing Information
Fulvestrant injection is indicated for the treatment of:
- Hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer in postmenopausal women not previously treated with endocrine therapy,
or - HR-positive advanced breast cancer in postmenopausal women with disease progression following endocrine therapy.
Fulvestrant injection is indicated for the treatment of:
- HR-positive, HER2-negative advanced or metastatic breast cancer in postmenopausal women in combination with ribociclib as initial endocrine based therapy or following disease progression on endocrine therapy.
- HR-positive, HER2-negative advanced or metastatic breast cancer in combination with palbociclib or abemaciclib in women with disease progression after endocrine therapy.
Administer the injection according to the local guidelines for performing large volume intramuscular injections.
NOTE: Due to the proximity of the underlying sciatic nerve, caution should be taken if administering fulvestrant injection at the dorsogluteal injection site
Injection site related events including sciatica, neuralgia, neuropathic pain, and peripheral neuropathy have been reported with fulvestrant injection. Caution should be taken while administering fulvestrant injection at the dorsogluteal injection site due to the proximity of the underlying sciatic nerve
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other trials and may not reflect the rates observed in clinical practice.
The following adverse reactions (ARs) were calculated based on the safety analysis of CONFIRM comparing the administration of fulvestrant injection 500 mg intramuscularly once a month with fulvestrant injection 250 mg intramuscularly once a month. The most frequently reported adverse reactions in the fulvestrant injection 500 mg group were injection site pain (11.6% of patients), nausea (9.7% of patients), and bone pain (9.4% of patients); the most frequently reported adverse reactions in the fulvestrant injection 250 mg group were nausea (13.6% of patients), back pain (10.7% of patients), and injection site pain (9.1% of patients).
Table 1 lists adverse reactions reported with an incidence of 5% or greater, regardless of assessed causality, from CONFIRM.
*Including more severe injection site related sciatica, neuralgia, neuropathic pain, and peripheral neuropathy. | ||
Adverse Reactions | Fulvestrant injection 500 mg N=361 % | Fulvestrant injection 250 mg N=374 % |
Body as a Whole | ||
| Injection Site Pain* | 12 | 9 |
| Headache | 8 | 7 |
| Back Pain | 8 | 11 |
| Fatigue | 8 | 6 |
| Pain in Extremity | 7 | 7 |
| Asthenia | 6 | 6 |
Vascular System | ||
| Hot Flash | 7 | 6 |
Digestive System | ||
| Nausea | 10 | 14 |
| Vomiting | 6 | 6 |
| Anorexia | 6 | 4 |
| Constipation | 5 | 4 |
Musculoskeletal System | ||
| Bone Pain | 9 | 8 |
| Arthralgia | 8 | 8 |
| Musculoskeletal Pain | 6 | 3 |
Respiratory System | ||
| Cough | 5 | 5 |
| Dyspnea | 4 | 5 |
In the pooled safety population (N=1127) from clinical trials comparing fulvestrant injection 500 mg to fulvestrant injection 250 mg, post-baseline increases of ≥1 CTC grade in either AST, ALT, or alkaline phosphatase were observed in >15% of patients receiving fulvestrant injection. Grade 3-4 increases were observed in 1-2% of patients. The incidence and severity of increased hepatic enzymes (ALT, AST, ALP) did not differ between the 250 mg and the 500 mg fulvestrant injection arms.
The safety of fulvestrant injection 500 mg versus anastrozole 1 mg was evaluated in FALCON. The data described below reflect exposure to fulvestrant injection in 228 out of 460 patients with HR-positive advanced breast cancer in postmenopausal women not previously treated with endocrine therapy who received at least one (1) dose of treatment in FALCON.
Permanent discontinuation associated with an adverse reaction occurred in 4 of 228 (1.8%) patients receiving fulvestrant injection and in 3 of 232 (1.3%) patients receiving anastrozole. Adverse reactions leading to discontinuation for those patients receiving fulvestrant injection included drug hypersensitivity (0.9%), injection site hypersensitivity (0.4%), and elevated liver enzymes (0.4%).
The most common adverse reactions (≥10%) of any grade reported in patients in the fulvestrant injection arm were arthralgia, hot flash, fatigue, and nausea.
Adverse reactions reported in patients who received fulvestrant injection in FALCON at an incidence of ≥5% in either treatment arm are listed in Table 2, and laboratory abnormalities are listed in Table 3.
Adverse Reactions | Fulvestrant injection 500 mg N=228 | Anastrozole 1 mg N=232 | ||
All Grades % | Grade 3 or 4 % | All Grades % | Grade 3 or 4 % | |
Vascular Disorders | ||||
| Hot flash | 11 | 0 | 10 | 0 |
Gastrointestinal Disorders | ||||
| Nausea | 11 | 0 | 10 | <1 |
| Diarrhea | 6 | 0 | 6 | <1 |
Musculoskeletal and Connective Tissue Disorders | ||||
| Arthralgia | 17 | 0 | 10 | 0 |
| Myalgia | 7 | 0 | 3 | 0 |
| Pain in extremity | 6 | 0 | 4 | 0 |
| Back pain | 9 | <1 | 6 | 0 |
General Disorders and Administration Site Conditions | ||||
| Fatigue | 11 | <1 | 7 | <1 |
*In FALCON, post- baseline increases of ≥1 CTC grade in either AST, ALT, or alkaline phosphatase were observed in >10% of patients receiving Fulvestrant injection. Grade 3- 4 increases were observed in 1%- 3% of patients. | ||||
Laboratory Parameters | Fulvestrant injection 500 mg N=228 | Anastrozole 1 mg N=232 | ||
All Grades % | Grade 3 or 4 % | All Grades % | Grade 3 or 4 % | |
| Alanine aminotransferase increased (ALT) | 7 | 1 | 3 | 0 |
| Aspartate aminotransferase increased (AST) | 5 | 1 | 3 | <1 |
The most commonly reported adverse reactions in the fulvestrant injection and anastrozole treatment groups were gastrointestinal symptoms (including nausea, vomiting, constipation, diarrhea, and abdominal pain), headache, back pain, vasodilatation (hot flashes), and pharyngitis.
Injection site reactions with mild transient pain and inflammation were seen with fulvestrant injection and occurred in 7% of patients given the single 5 mL injection (Study 0020) and in 27% of patients given the 2 x 2.5 mL injections (Study 0021) in the two clinical trials that compared fulvestrant injection 250 mg and anastrozole 1 mg.
Table 4 lists adverse reactions reported with an incidence of 5% or greater, regardless of assessed causality, from the two controlled clinical trials comparing the administration of fulvestrant injection 250 mg intramuscularly once a month with anastrozole 1 mg orally once a day.
*Including more severe injection site related sciatica, neuralgia, neuropathic pain, and peripheral neuropathy. All patients on Fulvestrant injection received injections, but only those anastrozole patients who were in Study 0021 received placebo injections. | ||
Adverse Reactions | Fulvestrant injection 250 mg N=423 % | Anastrozole 1 mg N=423 % |
Body as a Whole | 68 | 68 |
| Asthenia | 23 | 27 |
| Pain | 19 | 20 |
| Headache | 15 | 17 |
| Back Pain | 14 | 13 |
| Abdominal Pain | 12 | 12 |
| Injection Site Pain* | 11 | 7 |
| Pelvic Pain | 10 | 9 |
| Chest Pain | 7 | 5 |
| Flu Syndrome | 7 | 6 |
| Fever | 6 | 6 |
| Accidental Injury | 5 | 6 |
Cardiovascular System | 30 | 28 |
| Vasodilatation | 18 | 17 |
Digestive System | 52 | 48 |
| Nausea | 26 | 25 |
| Vomiting | 13 | 12 |
| Constipation | 13 | 11 |
| Diarrhea | 12 | 13 |
| Anorexia | 9 | 11 |
Hemic and Lymphatic Systems | 14 | 14 |
| Anemia | 5 | 5 |
Metabolic and Nutritional Disorders | 18 | 18 |
| Peripheral Edema | 9 | 10 |
Musculoskeletal System | 26 | 28 |
| Bone Pain | 16 | 14 |
| Arthritis | 3 | 6 |
Nervous System | 34 | 34 |
| Dizziness | 7 | 7 |
| Insomnia | 7 | 9 |
| Paresthesia | 6 | 8 |
| Depression | 6 | 7 |
| Anxiety | 5 | 4 |
Respiratory System | 39 | 34 |
| Pharyngitis | 16 | 12 |
| Dyspnea | 15 | 12 |
| Cough Increased | 10 | 10 |
Skin and Appendages | 22 | 23 |
| Rash | 7 | 8 |
| Sweating | 5 | 5 |
Urogenital System | 18 | 15 |
| Urinary Tract Infection | 6 | 4 |
The safety of fulvestrant injection 500 mg plus palbociclib 125 mg/day versus fulvestrant injection plus placebo was evaluated in PALOMA-3. The data described below reflect exposure to fulvestrant injection plus palbociclib in 345 out of 517 patients with HR-positive, HER2-negative advanced or metastatic breast cancer who received at least 1 dose of treatment in PALOMA-3. The median duration of treatment for fulvestrant injection plus palbociclib was 10.8 months while the median duration of treatment for fulvestrant injection plus placebo arm was 4.8 months.
No dose reduction was allowed for fulvestrant injection in PALOMA-3. Dose reductions of palbociclib due to an adverse reaction of any grade occurred in 36% of patients receiving fulvestrant injection plus palbociclib.
Permanent discontinuation associated with an adverse reaction occurred in 19 of 345 (6%) patients receiving fulvestrant injection plus palbociclib, and in 6 of 172 (3%) patients receiving fulvestrant injection plus placebo. Adverse reactions leading to discontinuation for those patients receiving fulvestrant injection plus palbociclib included fatigue (0.6%), infections (0.6%), and thrombocytopenia (0.6%).
The most common adverse reactions (≥10%) of any grade reported in patients in the fulvestrant injection plus palbociclib arm by descending frequency were neutropenia, leukopenia, infections, fatigue, nausea, anemia, stomatitis, diarrhea, thrombocytopenia, vomiting, alopecia, rash, decreased appetite, and pyrexia.
The most frequently reported Grade ≥3 adverse reactions (≥5%) in patients receiving fulvestrant injection plus palbociclib in descending frequency were neutropenia and leukopenia.
Adverse reactions (≥10%) reported in patients who received fulvestrant injection plus palbociclib or fulvestrant injection plus placebo in PALOMA-3 are listed in Table 5, and laboratory abnormalities are listed in Table 6.
Grading according to CTCAE v.4.0. | ||||||
CTCAE=Common Terminology Criteria for Adverse Events; N=number of patients; N/A=not applicable. | ||||||
*Infections includes all reported preferred terms (PTs) that are part of the System Organ Class Infections and infestations. | ||||||
†Most common infections (≥1%) include: nasopharyngitis, upper respiratory infection, urinary tract infection, influenza, bronchitis, rhinitis, conjunctivitis, pneumonia, sinusitis, cystitis, oral herpes, respiratory tract infection, gastroenteritis, tooth infection, pharyngitis, eye infection, herpes simplex, paronychia. | ||||||
‡Stomatitis includes: aphthous stomatitis, cheilitis, glossitis, glossodynia, mouth ulceration, mucosal inflammation, oral pain, oropharyngeal discomfort, oropharyngeal pain, stomatitis. | ||||||
§Grade 1 events – 17%; Grade 2 events – 1%. | ||||||
¶Grade 1 events – 6%. | ||||||
#Rash includes: rash, rash maculo- papular, rash pruritic, rash erythematous, rash papular, dermatitis, dermatitis acneiform, toxic skin eruption. | ||||||
Adverse Reactions | Fulvestrant injection plus Palbociclib N=345 | Fulvestrant injection plus Placebo N=172 | ||||
All Grades % | Grade 3 % | Grade 4 % | All Grades % | Grade 3 % | Grade 4 % | |
Infections and Infestations | ||||||
| Infections* | 47† | 3 | 1 | 31 | 3 | 0 |
Blood and Lymphatic System Disorders | ||||||
| Neutropenia | 83 | 55 | 11 | 4 | 1 | 0 |
| Leukopenia | 53 | 30 | 1 | 5 | 1 | 1 |
| Anemia | 30 | 4 | 0 | 13 | 2 | 0 |
| Thrombocytopenia | 23 | 2 | 1 | 0 | 0 | 0 |
Metabolism and Nutrition Disorders | ||||||
| Decreased appetite | 16 | 1 | 0 | 8 | 1 | 0 |
Gastrointestinal Disorders | ||||||
| Nausea | 34 | 0 | 0 | 28 | 1 | 0 |
| Stomatitis‡ | 28 | 1 | 0 | 13 | 0 | 0 |
| Diarrhea | 24 | 0 | 0 | 19 | 1 | 0 |
| Vomiting | 19 | 1 | 0 | 15 | 1 | 0 |
Skin and Subcutaneous Tissue Disorders | ||||||
| Alopecia | 18§ | N/A | N/A | 6>¶ | N/A | N/A |
| Rash# | 17 | 1 | 0 | 6 | 0 | 0 |
General Disorders and Administration Site Conditions | ||||||
| Fatigue | 41 | 2 | 0 | 29 | 1 | 0 |
| Pyrexia | 13 | <1 | 0 | 5 | 0 | 0 |
Additional adverse reactions occurring at an overall incidence of <10.0% of patients receiving fulvestrant injection plus palbociclib in PALOMA-3 included asthenia (7.5%), aspartate aminotransferase increased (7.5%), dysgeusia (6.7%), epistaxis (6.7%), lacrimation increased (6.4%), dry skin (6.1%), alanine aminotransferase increased (5.8%), vision blurred (5.8%), dry eye (3.8%), and febrile neutropenia (0.9%).
N=number of patients; WBC=white blood cells. | ||||||
Laboratory Parameters | Fulvestrant injection plus Palbociclib N=345 | Fulvestrant injection plus Placebo N=172 | ||||
All Grades % | Grade 3 % | Grade 4 % | All Grades % | Grade 3 % | Grade 4 % | |
| WBC decreased | 99 | 45 | 1 | 26 | 0 | 1 |
| Neutrophils decreased | 96 | 56 | 11 | 14 | 0 | 1 |
| Anemia | 78 | 3 | 0 | 40 | 2 | 0 |
| Platelets decreased | 62 | 2 | 1 | 10 | 0 | 0 |
| Aspartate aminotransferase increased | 43 | 4 | 0 | 48 | 4 | 0 |
| Alanine aminotransferase increased | 36 | 2 | 0 | 34 | 0 | 0 |
The safety of fulvestrant injection (500 mg) plus abemaciclib (150 mg twice daily) versus fulvestrant injection plus placebo was evaluated in MONARCH 2. The data described below reflect exposure to fulvestrant injection in 664 patients with HR-positive, HER2-negative advanced breast cancer who received at least one dose of fulvestrant injection plus abemaciclib or placebo in MONARCH 2.
Median duration of treatment was 12 months for patients receiving fulvestrant injection plus abemaciclib and 8 months for patients receiving fulvestrant injection plus placebo.
Dose reductions due to an adverse reaction occurred in 43% of patients receiving fulvestrant injection plus abemaciclib. Adverse reactions leading to dose reductions ≥5% of patients were diarrhea and neutropenia. Abemaciclib dose reduction due to diarrhea of any grade occurred in 19% of patients receiving fulvestrant injection plus abemaciclib compared to 0.4% of patients receiving fulvestrant injection plus placebo. Abemaciclib dose reductions due to neutropenia of any grade occurred in 10% of patients receiving fulvestrant injection plus abemaciclib compared to no patients receiving fulvestrant injection plus placebo.
Permanent study treatment discontinuation due to an adverse event was reported in 9% of patients receiving fulvestrant injection plus abemaciclib and in 3% of patients receiving fulvestrant injection plus placebo. Adverse reactions leading to permanent discontinuation for patients receiving fulvestrant injection plus abemaciclib were infection (2%), diarrhea (1%), hepatotoxicity (1%), fatigue (0.7%), nausea (0.2%), abdominal pain (0.2%), acute kidney injury (0.2%), and cerebral infarction (0.2%).
Deaths during treatment or during the 30-day follow up, regardless of causality, were reported in 18 cases (4%) of fulvestrant injection plus abemaciclib treated patients versus 10 cases (5%) of Fulvestrant injection plus placebo treated patients. Causes of death for patients receiving Fulvestrant injection plus abemaciclib included: 7 (2%) patient deaths due to underlying disease, 4 (0.9%) due to sepsis, 2 (0.5%) due to pneumonitis, 2 (0.5%) due to hepatotoxicity, and one (0.2%) due to cerebral infarction.
The most common adverse reactions reported (≥20%) in the fulvestrant injection plus abemaciclib arm were diarrhea, fatigue, neutropenia, nausea, infections, abdominal pain, anemia, leukopenia, decreased appetite, vomiting, and headache (Table 7). The most frequently reported (≥5%) Grade 3 or 4 adverse reactions were neutropenia, diarrhea, leukopenia, anemia, and infections.
*Includes abdominal pain, abdominal pain upper, abdominal pain lower, abdominal discomfort, abdominal tenderness. | ||||||
†Includes upper respiratory tract infection, urinary tract infection, lung infection, pharyngitis, conjunctivitis, sinusitis, vaginal infection, sepsis. | ||||||
‡Includes neutropenia, neutrophil count decreased. | ||||||
§Includes anemia, hematocrit decreased, hemoglobin decreased, red blood cell count decreased. | ||||||
¶Includes leukopenia, white blood cell count decreased. | ||||||
#Includes platelet count decreased, thrombocytopenia. | ||||||
ÞIncludes asthenia, fatigue. | ||||||
Adverse Reactions | Fulvestrant injection plus Abemaciclib N=441 | Fulvestrant injection plus Placebo N=223 | ||||
All Grades % | Grade 3 % | Grade 4 % | All Grades % | Grade 3 % | Grade 4 % | |
Gastrointestinal Disorders | ||||||
| Diarrhea | 86 | 13 | 0 | 25 | <1 | 0 |
| Nausea | 45 | 3 | 0 | 23 | 1 | 0 |
| Abdominal pain* | 35 | 2 | 0 | 16 | 1 | 0 |
| Vomiting | 26 | <1 | 0 | 10 | 2 | 0 |
| Stomatitis | 15 | <1 | 0 | 10 | 0 | 0 |
Infections and Infestations | ||||||
| Infections† | 43 | 5 | <1 | 25 | 3 | <1 |
Blood and Lymphatic System Disorders | ||||||
| Neutropenia‡ | 46 | 24 | 3 | 4 | 1 | <1 |
| Anemia§ | 29 | 7 | <1 | 4 | 1 | 0 |
| Leukopenia¶ | 28 | 9 | <1 | 2 | 0 | 0 |
| Thrombocytopenia# | 16 | 2 | 1 | 3 | 0 | <1 |
General Disorders and Administration Site Conditions | ||||||
| FatigueÞ | 46 | 3 | 0 | 32 | <1 | 0 |
| Edema peripheral | 12 | 0 | 0 | 7 | 0 | 0 |
| Pyrexia | 11 | <1 | <1 | 6 | <1 | 0 |
Metabolism and Nutrition Disorders | ||||||
| Decreased appetite | 27 | 1 | 0 | 12 | <1 | 0 |
Respiratory, Thoracic, and Mediastinal Disorders | ||||||
| Cough | 13 | 0 | 0 | 11 | 0 | 0 |
Skin and Subcutaneous Tissue Disorders | ||||||
| Alopecia | 16 | 0 | 0 | 2 | 0 | 0 |
| Pruritus | 13 | 0 | 0 | 6 | 0 | 0 |
| Rash | 11 | 1 | 0 | 4 | 0 | 0 |
Nervous System Disorders | ||||||
| Headache | 20 | 1 | 0 | 15 | <1 | 0 |
| Dysgeusia | 18 | 0 | 0 | 3 | 0 | 0 |
| Dizziness | 12 | 1 | 0 | 6 | 0 | 0 |
Investigations | ||||||
| Alanine aminotransferase increased | 13 | 4 | <1 | 5 | 2 | 0 |
| Aspartate aminotransferase increased | 12 | 2 | 0 | 7 | 3 | 0 |
| Creatinine increased | 12 | <1 | 0 | <1 | 0 | 0 |
| Weight decreased | 10 | <1 | 0 | 2 | <1 | 0 |
Additional adverse reactions in MONARCH 2 include venous thromboembolic events (deep vein thrombosis, pulmonary embolism, cerebral venous sinus thrombosis, subclavian vein thrombosis, axillary vein thrombosis, and DVT inferior vena cava), which were reported in 5% of patients treated with fulvestrant injection plus abemaciclib as compared to 0.9% of patients treated with fulvestrant injection plus placebo.
Laboratory Parameters | Fulvestrant plus Abemaciclib N=441 | Fulvestrant plus Placebo N=223 | ||||
All Grades % | Grade 3 % | Grade 4 % | All Grades % | Grade 3 % | Grade 4 % | |
| Creatinine increased | 98 | 1 | 0 | 74 | 0 | 0 |
| White blood cell decreased | 90 | 23 | <1 | 33 | <1 | 0 |
| Neutrophil count decreased | 87 | 29 | 4 | 30 | 4 | <1 |
| Anemia | 84 | 3 | 0 | 33 | <1 | 0 |
| Lymphocyte count decreased | 63 | 12 | <1 | 32 | 2 | 0 |
| Platelet count decreased | 53 | <1 | 1 | 15 | 0 | 0 |
| Alanine aminotransferase increased | 41 | 4 | <1 | 32 | 1 | 0 |
| Aspartate aminotransferase increased | 37 | 4 | 0 | 25 | 4 | <1 |
The safety of fulvestrant injection 500 mg plus ribociclib 600 mg versus fulvestrant injection plus placebo was evaluated in MONALEESA-3. The data described below reflect exposure to fulvestrant injection plus ribociclib in 483 out of 724 postmenopausal patients with HRpositive, HER2-negative advanced or metastatic breast cancer for initial endocrine based therapy or after disease progression on endocrine therapy who received at least one dose of fulvestrant injection plus ribociclib or placebo in MONALEESA-3. Median duration of treatment was 15.8 months for fulvestrant injection plus ribociclib and 12 months for fulvestrant injection plus placebo.
Dose reductions due to adverse reactions occurred in 32% of patients receiving fulvestrant injection plus ribociclib and in 3% of patients receiving fulvestrant injection plus placebo. Among patients receiving fulvestrant injection plus ribociclib, 8% were reported to have permanently discontinued both fulvestrant injection plus ribociclib, and 9% were reported to have discontinued ribociclib alone due to ARs. Among patients receiving fulvestrant injection plus placebo, 4% were reported to have permanently discontinued both fulvestrant injection and placebo and 2% were reported to have discontinued placebo alone due to ARs.
Adverse reactions leading to treatment discontinuation of fulvestrant injection plus ribociclib (as compared to fulvestrant injection plus placebo) were ALT increased (5% vs. 0%), AST increased (3% vs. 0.6%), and vomiting (1% vs. 0%).
The most common adverse reactions (reported at a frequency ≥20% on the fulvestrant injection plus ribociclib arm and ≥2% higher than fulvestrant injection plus placebo) were neutropenia, infections, leukopenia, cough, nausea, diarrhea, vomiting, constipation, pruritus, and rash. The most frequently reported Grade 3/4 adverse reactions (reported at a frequency ≥5%) in patients receiving fulvestrant injection plus ribociclib in descending frequency were neutropenia, leukopenia, infections, and abnormal liver function tests.
Adverse reactions and laboratory abnormalities occurring in patients in MONALEESA-3 are listed in Table 9 and Table 10, respectively.
Grading according to CTCAE 4.03. | ||||||
CTCAE=Common Terminology Criteria for Adverse Events; N=number of patients | ||||||
*Infections; urinary tract infections; respiratory tract infections; gastroenteritis; sepsis (<1%). | ||||||
Adverse Reactions | Fulvestrant injection plus Ribociclib N=483 | Fulvestrant injection plus Placebo N=241 | ||||
All Grades % | Grade 3 % | Grade 4 % | All Grades % | Grade 3 % | Grade 4 % | |
Infections and Infestations | ||||||
| Infections* | 42 | 5 | 0 | 30 | 2 | 0 |
Blood and Lymphatic System Disorders | ||||||
| Neutropenia | 69 | 46 | 7 | 2 | 0 | 0 |
| Leukopenia | 27 | 12 | <1 | <1 | 0 | 0 |
| Anemia | 17 | 3 | 0 | 5 | 2 | 0 |
Metabolism and Nutrition Disorders | ||||||
| Decreased appetite | 16 | <1 | 0 | 13 | 0 | 0 |
Nervous System Disorders | ||||||
| Dizziness | 13 | <1 | 0 | 8 | 0 | 0 |
Respiratory, Thoracic, and Mediastinal Disorders | ||||||
| Cough | 22 | 0 | 0 | 15 | 0 | 0 |
| Dyspnea | 15 | 1 | <1 | 12 | 2 | 0 |
Gastrointestinal Disorders | ||||||
| Nausea | 45 | 1 | 0 | 28 | <1 | 0 |
| Diarrhea | 29 | <1 | 0 | 20 | <1 | 0 |
| Vomiting | 27 | 1 | 0 | 13 | 0 | 0 |
| Constipation | 25 | <1 | 0 | 12 | 0 | 0 |
| Abdominal pain | 17 | 1 | 0 | 13 | <1 | 0 |
Skin and Subcutaneous Tissue Disorders | ||||||
| Alopecia | 19 | 0 | 0 | 5 | 0 | 0 |
| Pruritus | 20 | <1 | 0 | 7 | 0 | 0 |
| Rash | 23 | <1 | 0 | 7 | 0 | 0 |
General Disorders and Administration Site Conditions | ||||||
| Edema peripheral | 15 | 0 | 0 | 7 | 0 | 0 |
| Pyrexia | 11 | <1 | 0 | 7 | 0 | 0 |
Investigations | ||||||
| Alanine aminotransferase increased | 15 | 7 | 2 | 5 | <1 | 0 |
| Aspartate aminotransferase increased | 13 | 5 | 1 | 5 | <1 | 0 |
Additional adverse reactions in MONALEESA-3 for patients receiving fulvestrant injection plus ribociclib included asthenia (14%), dyspepsia (10%), thrombocytopenia (9%), dry skin (8%), dysgeusia (7%), electrocardiogram QT prolonged (6%), dry mouth (5%), vertigo (5%), dry eye (5%), lacrimation increased (4%), erythema (4%), hypocalcemia (4%), blood bilirubin increased (1%), and syncope (1%).
Laboratory parameters | Fulvestrant injection plus Ribociclib N=483 | Fulvestrant injection plus Placebo N=241 | ||||
All Grades % | Grade 3 % | Grade 4 % | All Grades % | Grade 3 % | Grade 4 % | |
Hematology | ||||||
| Leukocyte count decreased | 95 | 25 | <1 | 26 | <1 | 0 |
| Neutrophil count decreased | 92 | 46 | 7 | 21 | <1 | 0 |
| Hemoglobin decreased | 60 | 4 | 0 | 35 | 3 | 0 |
| Lymphocyte count decreased | 69 | 14 | 1 | 35 | 4 | <1 |
| Platelet count decreased | 33 | <1 | 1 | 11 | 0 | 0 |
Chemistry | ||||||
| Creatinine increased | 65 | <1 | <1 | 33 | <1 | 0 |
| Gamma-glutamyl transferase increased | 52 | 6 | 1 | 49 | 8 | 2 |
| Aspartate aminotransferase increased | 49 | 5 | 2 | 43 | 3 | 0 |
| Alanine aminotransferase increased | 44 | 8 | 3 | 37 | 2 | 0 |
| Glucose serum decreased | 23 | 0 | 0 | 18 | 0 | 0 |
| Phosphorous decreased | 18 | 5 | 0 | 8 | <1 | 0 |
| Albumin decreased | 12 | 0 | 0 | 8 | 0 | 0 |
The proper method of administration of fulvestrant injection for intramuscular use is described in the following instructions.
For each single-dose prefilled syringe:
- Remove glass syringe barrel from tray and check that it is not damaged.
- Remove perforated patient record label from syringe.
- Inspect drug product in glass syringe for any visible particulate matter or discoloration prior to use. Discard if particulate matter or discoloration is present.
- Peel open the safety needle (SafetyGlide™) outer packaging.
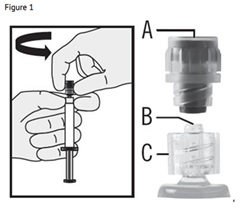
- Hold the syringe upright on the ribbed part (C). With the other hand, take hold of the cap (A) and carefully TWIST THE CAP COUNTER-CLOCKWISE until the cap disconnects for removal (see Figure 1).
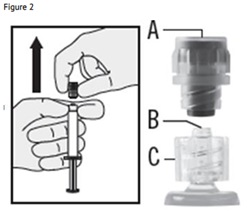
6. Pull the cap (A) off in a straight upward direction. DO NOT TOUCH THE STERILE SYRINGE TIP (Luer-Lok) (B) (see Figure 2).
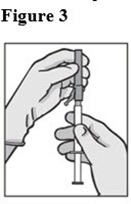
7. Attach the safety needle to the syringe tip (Luer-Lok). Twist needle until firmly seated (see Figure 3). Confirm that the needle is locked to the Luer connector before moving or tilting the syringe out of the vertical plane to avoid spillage of syringe contents.
For Administration:
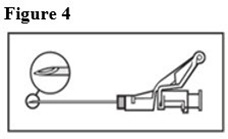
8. Pull shield straight off needle to avoid damaging needle point.9. Remove needle sheath.10. Expel excess gas from the syringe (a small gas bubble may remain).11. Administer intramuscularly slowly (1-2 minutes/injection) into the buttock (gluteal area). For user convenience, the needle ‘bevel up’ position is orientated to the lever arm, as shown in Figure 4.
NOTE: Activate away from self and others.
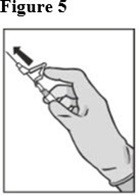
For the 2 x 5 mL syringe package, the contents of both syringes must be injected to receive the 500 mg recommended dose.
SafetyGlide™ is a trademark of Becton Dickinson and Company.
To help avoid HIV (AIDS), HBV (Hepatitis), and other infectious diseases due to accidental needlesticks, contaminated needles should not be recapped or removed, unless there is no alternative or that such action is required by a specific medical procedure. Hands must remain behind the needle at all times during use and disposal.
Do not autoclave SafetyGlide™ Needle before use.
Becton Dickinson guarantees the contents of their unopened or undamaged packages to be sterile, non- toxic, and non-pyrogenic.
Fulvestrant injection, an injection for intramuscular administration, is supplied as 5-mL single-dose prefilled syringes containing 250 mg/5 mL fulvestrant.
Negligible amounts of fulvestrant are eliminated in urine; therefore, a study in patients with renal impairment was not conducted. In the advanced breast cancer trials, fulvestrant concentrations in women with estimated creatinine clearance as low as 30 mL/min were similar to women with normal creatinine.
Fulvestrant injection is contraindicated in patients with a known hypersensitivity to the drug or to any of its components. Hypersensitivity reactions, including urticaria and angioedema, have been reported in association with fulvestrant injection
The following adverse reactions have been identified during post-approval use of fulvestrant injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
For fulvestrant injection 250 mg, other adverse reactions reported as drug-related and seen infrequently (<1%) include thromboembolic phenomena, myalgia, vertigo, leukopenia, and hypersensitivity reactions, including angioedema and urticaria.
Vaginal bleeding has been reported infrequently (<1%), mainly in patients during the first 6 weeks after changing from existing hormonal therapy to treatment with fulvestrant injection. If bleeding persists, further evaluation should be considered.
Elevation of bilirubin, elevation of gamma GT, hepatitis, and liver failure have been reported infrequently (<1%).
Due to structural similarity of fulvestrant and estradiol, Fulvestrant injection can interfere with estradiol measurement by immunoassay, resulting in falsely elevated estradiol levels.