Fycompa Prescribing Information
- Serious or life-threatening psychiatric and behavioral adverse reactions including aggression, hostility, irritability, anger, and homicidal ideation and threats have been reported in patients taking FYCOMPA ().
5.1 Serious Psychiatric and Behavioral ReactionsIn the controlled partial-onset seizure clinical trials, hostility- and aggression-related adverse reactions occurred in 12% and 20% of patients randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 6% of patients in the placebo group. These effects were dose-related and generally appeared within the first 6 weeks of treatment, although new events continued to be observed through more than 37 weeks. FYCOMPA-treated patients experienced more hostility- and aggression-related adverse reactions that were serious, severe, and led to dose reduction, interruption, and discontinuation more frequently than placebo-treated patients.
In general, in placebo-controlled partial-onset seizure clinical trials, neuropsychiatric events were reported more frequently in patients being treated with FYCOMPA than in patients taking placebo. These events included irritability, aggression, anger, and anxiety, which occurred in 2% or greater of FYCOMPA-treated patients and twice as frequently as in placebo-treated patients. Other symptoms that occurred with FYCOMPA and were more common than with placebo included belligerence, affect lability, agitation, and physical assault. Some of these events were reported as serious and life-threatening. Homicidal ideation and/or threat were exhibited in 0.1% of 4,368 FYCOMPA-treated patients in controlled and open label trials, including non-epilepsy trials
.Homicidal ideation and/or threat have also been reported postmarketing in patients treated with FYCOMPA.In the partial-onset seizure clinical trials, these events occurred in patients with and without prior psychiatric history, prior aggressive behavior, or concomitant use of medications associated with hostility and aggression. Some patients experienced worsening of their pre-existing psychiatric conditions. Patients with active psychotic disorders and unstable recurrent affective disorders were excluded from the clinical trials. The combination of alcohol and FYCOMPA significantly worsened mood and increased anger. Patients taking FYCOMPA should avoid the use of alcohol
[see Drug Interactions (7.3)].Similar serious psychiatric and behavioral events were observed in the primary generalized tonic-clonic seizure clinical trial.
In healthy volunteers taking FYCOMPA, observed psychiatric events included paranoia, euphoric mood, agitation, anger, mental status changes, and disorientation/confusional state.
In the non-epilepsy trials, psychiatric events that occurred in perampanel-treated patients more often than placebo-treated patients included disorientation, delusion, and paranoia.
In the postmarketing setting, there have been reports of psychosis (acute psychosis, hallucinations, delusions, paranoia) and delirium (delirium, confusional state, disorientation, memory impairment) in patients treated with FYCOMPA
[see Adverse Reactions (6.2)].Patients, their caregivers, and families should be informed that FYCOMPA may increase the risk of psychiatric events. Patients should be monitored during treatment and for at least 1 month after the last dose of FYCOMPA, and especially when taking higher doses and during the initial few weeks of drug therapy (titration period) or at other times of dose increases. Dose of FYCOMPA should be reduced if these symptoms occur. Permanently discontinue FYCOMPA for persistent severe or worsening psychiatric symptoms or behaviors and refer for psychiatric evaluation.
- These reactions occurred in patients with and without prior psychiatric history, prior aggressive behavior, or concomitant use of medications associated with hostility and aggression ().
5.1 Serious Psychiatric and Behavioral ReactionsIn the controlled partial-onset seizure clinical trials, hostility- and aggression-related adverse reactions occurred in 12% and 20% of patients randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 6% of patients in the placebo group. These effects were dose-related and generally appeared within the first 6 weeks of treatment, although new events continued to be observed through more than 37 weeks. FYCOMPA-treated patients experienced more hostility- and aggression-related adverse reactions that were serious, severe, and led to dose reduction, interruption, and discontinuation more frequently than placebo-treated patients.
In general, in placebo-controlled partial-onset seizure clinical trials, neuropsychiatric events were reported more frequently in patients being treated with FYCOMPA than in patients taking placebo. These events included irritability, aggression, anger, and anxiety, which occurred in 2% or greater of FYCOMPA-treated patients and twice as frequently as in placebo-treated patients. Other symptoms that occurred with FYCOMPA and were more common than with placebo included belligerence, affect lability, agitation, and physical assault. Some of these events were reported as serious and life-threatening. Homicidal ideation and/or threat were exhibited in 0.1% of 4,368 FYCOMPA-treated patients in controlled and open label trials, including non-epilepsy trials
.Homicidal ideation and/or threat have also been reported postmarketing in patients treated with FYCOMPA.In the partial-onset seizure clinical trials, these events occurred in patients with and without prior psychiatric history, prior aggressive behavior, or concomitant use of medications associated with hostility and aggression. Some patients experienced worsening of their pre-existing psychiatric conditions. Patients with active psychotic disorders and unstable recurrent affective disorders were excluded from the clinical trials. The combination of alcohol and FYCOMPA significantly worsened mood and increased anger. Patients taking FYCOMPA should avoid the use of alcohol
[see Drug Interactions (7.3)].Similar serious psychiatric and behavioral events were observed in the primary generalized tonic-clonic seizure clinical trial.
In healthy volunteers taking FYCOMPA, observed psychiatric events included paranoia, euphoric mood, agitation, anger, mental status changes, and disorientation/confusional state.
In the non-epilepsy trials, psychiatric events that occurred in perampanel-treated patients more often than placebo-treated patients included disorientation, delusion, and paranoia.
In the postmarketing setting, there have been reports of psychosis (acute psychosis, hallucinations, delusions, paranoia) and delirium (delirium, confusional state, disorientation, memory impairment) in patients treated with FYCOMPA
[see Adverse Reactions (6.2)].Patients, their caregivers, and families should be informed that FYCOMPA may increase the risk of psychiatric events. Patients should be monitored during treatment and for at least 1 month after the last dose of FYCOMPA, and especially when taking higher doses and during the initial few weeks of drug therapy (titration period) or at other times of dose increases. Dose of FYCOMPA should be reduced if these symptoms occur. Permanently discontinue FYCOMPA for persistent severe or worsening psychiatric symptoms or behaviors and refer for psychiatric evaluation.
- Advise patients and caregivers to contact a healthcare provider immediately if any of these reactions or changes in mood, behavior, or personality that are not typical for the patient are observed while taking FYCOMPA or after discontinuing FYCOMPA ().
5.1 Serious Psychiatric and Behavioral ReactionsIn the controlled partial-onset seizure clinical trials, hostility- and aggression-related adverse reactions occurred in 12% and 20% of patients randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 6% of patients in the placebo group. These effects were dose-related and generally appeared within the first 6 weeks of treatment, although new events continued to be observed through more than 37 weeks. FYCOMPA-treated patients experienced more hostility- and aggression-related adverse reactions that were serious, severe, and led to dose reduction, interruption, and discontinuation more frequently than placebo-treated patients.
In general, in placebo-controlled partial-onset seizure clinical trials, neuropsychiatric events were reported more frequently in patients being treated with FYCOMPA than in patients taking placebo. These events included irritability, aggression, anger, and anxiety, which occurred in 2% or greater of FYCOMPA-treated patients and twice as frequently as in placebo-treated patients. Other symptoms that occurred with FYCOMPA and were more common than with placebo included belligerence, affect lability, agitation, and physical assault. Some of these events were reported as serious and life-threatening. Homicidal ideation and/or threat were exhibited in 0.1% of 4,368 FYCOMPA-treated patients in controlled and open label trials, including non-epilepsy trials
.Homicidal ideation and/or threat have also been reported postmarketing in patients treated with FYCOMPA.In the partial-onset seizure clinical trials, these events occurred in patients with and without prior psychiatric history, prior aggressive behavior, or concomitant use of medications associated with hostility and aggression. Some patients experienced worsening of their pre-existing psychiatric conditions. Patients with active psychotic disorders and unstable recurrent affective disorders were excluded from the clinical trials. The combination of alcohol and FYCOMPA significantly worsened mood and increased anger. Patients taking FYCOMPA should avoid the use of alcohol
[see Drug Interactions (7.3)].Similar serious psychiatric and behavioral events were observed in the primary generalized tonic-clonic seizure clinical trial.
In healthy volunteers taking FYCOMPA, observed psychiatric events included paranoia, euphoric mood, agitation, anger, mental status changes, and disorientation/confusional state.
In the non-epilepsy trials, psychiatric events that occurred in perampanel-treated patients more often than placebo-treated patients included disorientation, delusion, and paranoia.
In the postmarketing setting, there have been reports of psychosis (acute psychosis, hallucinations, delusions, paranoia) and delirium (delirium, confusional state, disorientation, memory impairment) in patients treated with FYCOMPA
[see Adverse Reactions (6.2)].Patients, their caregivers, and families should be informed that FYCOMPA may increase the risk of psychiatric events. Patients should be monitored during treatment and for at least 1 month after the last dose of FYCOMPA, and especially when taking higher doses and during the initial few weeks of drug therapy (titration period) or at other times of dose increases. Dose of FYCOMPA should be reduced if these symptoms occur. Permanently discontinue FYCOMPA for persistent severe or worsening psychiatric symptoms or behaviors and refer for psychiatric evaluation.
- Closely monitor patients particularly during the titration period and at higher doses ().
5.1 Serious Psychiatric and Behavioral ReactionsIn the controlled partial-onset seizure clinical trials, hostility- and aggression-related adverse reactions occurred in 12% and 20% of patients randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 6% of patients in the placebo group. These effects were dose-related and generally appeared within the first 6 weeks of treatment, although new events continued to be observed through more than 37 weeks. FYCOMPA-treated patients experienced more hostility- and aggression-related adverse reactions that were serious, severe, and led to dose reduction, interruption, and discontinuation more frequently than placebo-treated patients.
In general, in placebo-controlled partial-onset seizure clinical trials, neuropsychiatric events were reported more frequently in patients being treated with FYCOMPA than in patients taking placebo. These events included irritability, aggression, anger, and anxiety, which occurred in 2% or greater of FYCOMPA-treated patients and twice as frequently as in placebo-treated patients. Other symptoms that occurred with FYCOMPA and were more common than with placebo included belligerence, affect lability, agitation, and physical assault. Some of these events were reported as serious and life-threatening. Homicidal ideation and/or threat were exhibited in 0.1% of 4,368 FYCOMPA-treated patients in controlled and open label trials, including non-epilepsy trials
.Homicidal ideation and/or threat have also been reported postmarketing in patients treated with FYCOMPA.In the partial-onset seizure clinical trials, these events occurred in patients with and without prior psychiatric history, prior aggressive behavior, or concomitant use of medications associated with hostility and aggression. Some patients experienced worsening of their pre-existing psychiatric conditions. Patients with active psychotic disorders and unstable recurrent affective disorders were excluded from the clinical trials. The combination of alcohol and FYCOMPA significantly worsened mood and increased anger. Patients taking FYCOMPA should avoid the use of alcohol
[see Drug Interactions (7.3)].Similar serious psychiatric and behavioral events were observed in the primary generalized tonic-clonic seizure clinical trial.
In healthy volunteers taking FYCOMPA, observed psychiatric events included paranoia, euphoric mood, agitation, anger, mental status changes, and disorientation/confusional state.
In the non-epilepsy trials, psychiatric events that occurred in perampanel-treated patients more often than placebo-treated patients included disorientation, delusion, and paranoia.
In the postmarketing setting, there have been reports of psychosis (acute psychosis, hallucinations, delusions, paranoia) and delirium (delirium, confusional state, disorientation, memory impairment) in patients treated with FYCOMPA
[see Adverse Reactions (6.2)].Patients, their caregivers, and families should be informed that FYCOMPA may increase the risk of psychiatric events. Patients should be monitored during treatment and for at least 1 month after the last dose of FYCOMPA, and especially when taking higher doses and during the initial few weeks of drug therapy (titration period) or at other times of dose increases. Dose of FYCOMPA should be reduced if these symptoms occur. Permanently discontinue FYCOMPA for persistent severe or worsening psychiatric symptoms or behaviors and refer for psychiatric evaluation.
- FYCOMPA should be reduced if these symptoms occur and should be discontinued immediately if symptoms are severe or are worsening ().
5.1 Serious Psychiatric and Behavioral ReactionsIn the controlled partial-onset seizure clinical trials, hostility- and aggression-related adverse reactions occurred in 12% and 20% of patients randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 6% of patients in the placebo group. These effects were dose-related and generally appeared within the first 6 weeks of treatment, although new events continued to be observed through more than 37 weeks. FYCOMPA-treated patients experienced more hostility- and aggression-related adverse reactions that were serious, severe, and led to dose reduction, interruption, and discontinuation more frequently than placebo-treated patients.
In general, in placebo-controlled partial-onset seizure clinical trials, neuropsychiatric events were reported more frequently in patients being treated with FYCOMPA than in patients taking placebo. These events included irritability, aggression, anger, and anxiety, which occurred in 2% or greater of FYCOMPA-treated patients and twice as frequently as in placebo-treated patients. Other symptoms that occurred with FYCOMPA and were more common than with placebo included belligerence, affect lability, agitation, and physical assault. Some of these events were reported as serious and life-threatening. Homicidal ideation and/or threat were exhibited in 0.1% of 4,368 FYCOMPA-treated patients in controlled and open label trials, including non-epilepsy trials
.Homicidal ideation and/or threat have also been reported postmarketing in patients treated with FYCOMPA.In the partial-onset seizure clinical trials, these events occurred in patients with and without prior psychiatric history, prior aggressive behavior, or concomitant use of medications associated with hostility and aggression. Some patients experienced worsening of their pre-existing psychiatric conditions. Patients with active psychotic disorders and unstable recurrent affective disorders were excluded from the clinical trials. The combination of alcohol and FYCOMPA significantly worsened mood and increased anger. Patients taking FYCOMPA should avoid the use of alcohol
[see Drug Interactions (7.3)].Similar serious psychiatric and behavioral events were observed in the primary generalized tonic-clonic seizure clinical trial.
In healthy volunteers taking FYCOMPA, observed psychiatric events included paranoia, euphoric mood, agitation, anger, mental status changes, and disorientation/confusional state.
In the non-epilepsy trials, psychiatric events that occurred in perampanel-treated patients more often than placebo-treated patients included disorientation, delusion, and paranoia.
In the postmarketing setting, there have been reports of psychosis (acute psychosis, hallucinations, delusions, paranoia) and delirium (delirium, confusional state, disorientation, memory impairment) in patients treated with FYCOMPA
[see Adverse Reactions (6.2)].Patients, their caregivers, and families should be informed that FYCOMPA may increase the risk of psychiatric events. Patients should be monitored during treatment and for at least 1 month after the last dose of FYCOMPA, and especially when taking higher doses and during the initial few weeks of drug therapy (titration period) or at other times of dose increases. Dose of FYCOMPA should be reduced if these symptoms occur. Permanently discontinue FYCOMPA for persistent severe or worsening psychiatric symptoms or behaviors and refer for psychiatric evaluation.
- 2 mg tablets: orange, round, debossed with “2” on one side and “Є 275” on the other.
- 4 mg tablets: red, round, debossed with “4” on one side and “Є 277” on the other.
- 6 mg tablets: pink, round, debossed with “6” on one side and “Є 294” on the other.
- 8 mg tablets: purple, round, debossed with “8” on one side and “Є 295” on the other.
- 10 mg tablets: green, round, debossed with “10” on one side and “Є 296” on the other.
- 12 mg tablets: blue, round, debossed with “12” on one side and “Є 297” on the other.
0.5 mg/mL white to off-white opaque liquid suspension for oral administration.
None.
The following serious adverse reactions are described below and elsewhere in the labeling:
- Serious Psychiatric and Behavioral Reactions [see]
5.1 Serious Psychiatric and Behavioral ReactionsIn the controlled partial-onset seizure clinical trials, hostility- and aggression-related adverse reactions occurred in 12% and 20% of patients randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 6% of patients in the placebo group. These effects were dose-related and generally appeared within the first 6 weeks of treatment, although new events continued to be observed through more than 37 weeks. FYCOMPA-treated patients experienced more hostility- and aggression-related adverse reactions that were serious, severe, and led to dose reduction, interruption, and discontinuation more frequently than placebo-treated patients.
In general, in placebo-controlled partial-onset seizure clinical trials, neuropsychiatric events were reported more frequently in patients being treated with FYCOMPA than in patients taking placebo. These events included irritability, aggression, anger, and anxiety, which occurred in 2% or greater of FYCOMPA-treated patients and twice as frequently as in placebo-treated patients. Other symptoms that occurred with FYCOMPA and were more common than with placebo included belligerence, affect lability, agitation, and physical assault. Some of these events were reported as serious and life-threatening. Homicidal ideation and/or threat were exhibited in 0.1% of 4,368 FYCOMPA-treated patients in controlled and open label trials, including non-epilepsy trials
.Homicidal ideation and/or threat have also been reported postmarketing in patients treated with FYCOMPA.In the partial-onset seizure clinical trials, these events occurred in patients with and without prior psychiatric history, prior aggressive behavior, or concomitant use of medications associated with hostility and aggression. Some patients experienced worsening of their pre-existing psychiatric conditions. Patients with active psychotic disorders and unstable recurrent affective disorders were excluded from the clinical trials. The combination of alcohol and FYCOMPA significantly worsened mood and increased anger. Patients taking FYCOMPA should avoid the use of alcohol
[see Drug Interactions (7.3)].Similar serious psychiatric and behavioral events were observed in the primary generalized tonic-clonic seizure clinical trial.
In healthy volunteers taking FYCOMPA, observed psychiatric events included paranoia, euphoric mood, agitation, anger, mental status changes, and disorientation/confusional state.
In the non-epilepsy trials, psychiatric events that occurred in perampanel-treated patients more often than placebo-treated patients included disorientation, delusion, and paranoia.
In the postmarketing setting, there have been reports of psychosis (acute psychosis, hallucinations, delusions, paranoia) and delirium (delirium, confusional state, disorientation, memory impairment) in patients treated with FYCOMPA
[see Adverse Reactions (6.2)].Patients, their caregivers, and families should be informed that FYCOMPA may increase the risk of psychiatric events. Patients should be monitored during treatment and for at least 1 month after the last dose of FYCOMPA, and especially when taking higher doses and during the initial few weeks of drug therapy (titration period) or at other times of dose increases. Dose of FYCOMPA should be reduced if these symptoms occur. Permanently discontinue FYCOMPA for persistent severe or worsening psychiatric symptoms or behaviors and refer for psychiatric evaluation.
- Suicidal Behavior and Ideation [see]
5.2 Suicidal Behavior and IdeationAntiepileptic drugs (AEDs), including FYCOMPA, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI: 1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as 1 week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs
used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed.Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
Table 1. Risk by indication for antiepileptic drugs in the pooled analysis Indication
Placebo Patients
with Events per
1000 Patients
Drug Patients with
Events per 1000
patients
Relative Risk:
Incidence of Events
in drug Patients/
Incidence in Placebo
Patients
Risk Difference:
Additional Drug
Patients with Events
per 1000 Patients
Epilepsy
1.0
3.4
3.5
2.4
Psychiatric
5.7
8.5
1.5
2.9
Other
1.0
1.8
1.9
0.9
Total
2.4
4.3
1.8
1.9
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing FYCOMPA or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
- Neurologic Effects [see]
5.3 Neurologic EffectsDizziness and Gait DisturbanceFYCOMPA caused dose-related increases in events related to dizziness and disturbance in gait or coordination
[see Adverse Reactions (6.1)]. In the controlled partial-onset seizure clinical trials, dizziness and vertigo were reported in 35% and 47% of patients randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 10% of placebo-treated patients. The gait disturbance related events (including ataxia, gait disturbance, balance disorder, and abnormal coordination) were reported in 12% and 16% of patients randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 2% of placebo-treated patients. Elderly patients had an increased risk of these adverse reactions compared to younger adults and pediatric patients.These adverse reactions occurred mostly during the titration phase and led to discontinuation in 3% of FYCOMPA-treated patients compared to 1% of placebo-treated patients.
These adverse reactions were also observed in the primary generalized tonic-clonic seizure clinical trial.
Somnolence and FatigueFYCOMPA caused dose-dependent increases in somnolence and fatigue-related events (including fatigue, asthenia, and lethargy).
In the controlled partial-onset seizure clinical trials, 16% and 18% of patients randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, reported somnolence compared to 7% of placebo patients. In the controlled partial-onset seizure clinical trials, 12% and 15% of patients randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, reported fatigue-related events compared to 5% of placebo patients. Somnolence or fatigue-related events led to discontinuation in 2% of FYCOMPA-treated patients and 0.5% of placebo-treated patients. Elderly patients had an
increased risk of these adverse reactions compared to younger adults and pediatric patients.In the controlled partial-onset seizure clinical trials, these adverse reactions occurred mostly during the titration phase.
These adverse reactions were also observed in the primary generalized tonic-clonic seizure clinical trial.
Risk AmeliorationPrescribers should advise patients against engaging in hazardous activities requiring mental alertness, such as operating motor vehicles or dangerous machinery, until the effect of FYCOMPA is known. Patients should be carefully observed for signs of central nervous system (CNS) depression, such as somnolence and sedation, when FYCOMPA is used with other drugs with sedative properties because of potential additive effects.
- Falls [see]
5.4 FallsAn increased risk of falls, in some cases leading to serious injuries including head injuries and bone fracture, occurred in patients being treated with FYCOMPA (with and without concurrent seizures). In the controlled partial-onset seizure clinical trials, falls were reported in 5% and 10% of patients randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 3% of placebo-treated patients. Falls were reported as serious and led to discontinuation more frequently in FYCOMPA-treated patients than placebo-treated patients. Elderly patients had an increased risk of falls compared to younger adults and pediatric patients.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
[see]5.5 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan HypersensitivityDrug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as Multiorgan hypersensitivity, has been reported in patients taking antiepileptic drugs, including FYCOMPA. DRESS may be fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling, in association with other organ system involvement, such as hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis sometimes resembling an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its expression, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. FYCOMPA should be discontinued if an alternative etiology for the signs or symptoms cannot be established.
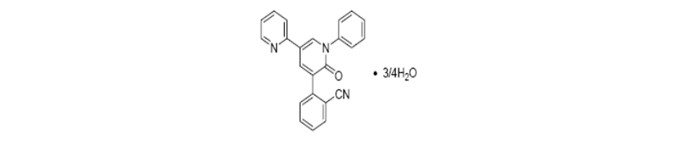
FYCOMPA tablets and oral suspension contain perampanel, a non-competitive AMPA receptor antagonist, as a 4:3 hydrate.
The chemical name of the active ingredient is 2-(1′,6′-dihydro-6′-oxo-1′-phenyl[2,3′-bipyridin]-5′-yl)-benzonitrile, hydrate (4:3).
The molecular formula is C23H15N3O • ¾H2O and the molecular weight is 362.90 (349.39 for anhydrous perampanel). It is a white to yellowish white powder. It is freely soluble in 1-methyl-2-pyrrolidinone, sparingly soluble in acetonitrile and acetone, slightly soluble in methanol, ethanol and ethyl acetate, very slightly soluble in 1-octanol and diethyl ether, and practically insoluble in heptane and water. The chemical structure is:
FYCOMPA tablets are round, bi-convex, film-coated tablets containing 2 mg, 4 mg, 6 mg, 8 mg, 10 mg, or 12 mg of perampanel. Tablets contain the following inactive ingredients: lactose monohydrate, low substituted hydroxypropyl cellulose, povidone, microcrystalline cellulose, magnesium stearate, hypromellose, polyethylene glycol, talc, and titanium dioxide. Tablets of different strengths may contain yellow ferric oxide (10 mg and 2 mg), red ferric oxide (2 mg, 4 mg, 6 mg, 8 mg), black ferric oxide (8 mg), and FD&C Blue No. 2 (indigo carmine) aluminum lake (10 mg and 12 mg).
FYCOMPA oral suspension is a white to off-white opaque liquid providing perampanel in a concentration of 0.5 mg/mL. The oral suspension contains the following inactive ingredients: sorbitol, microcrystalline cellulose, carboxymethyl-cellulose sodium, poloxamer, simethicone, citric acid, sodium benzoate and purified water.
Read this Instructions for Use before you start using FYCOMPA Oral Suspension and each time you get a refill. There
may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical
condition or treatment.
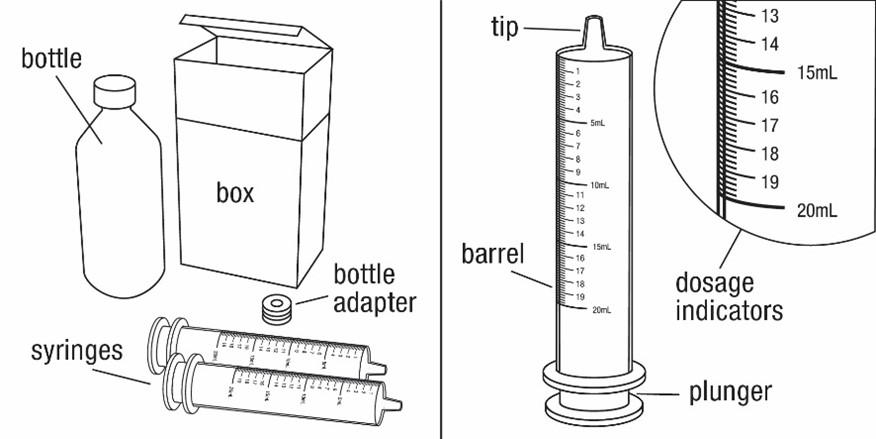
You will need the following supplies:
• FYCOMPA Oral Suspension bottle
• Bottle adapter
• Dosing syringe (2 dosing syringes are included in the FYCOMPA Oral Suspension box)
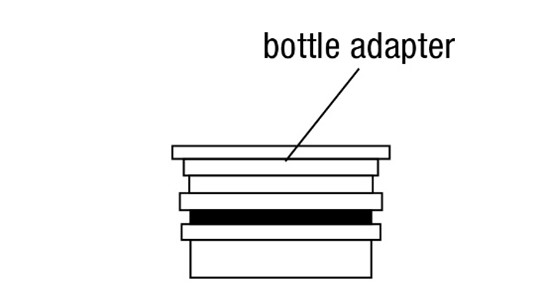
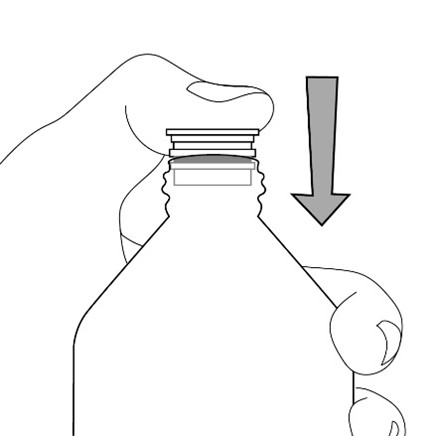
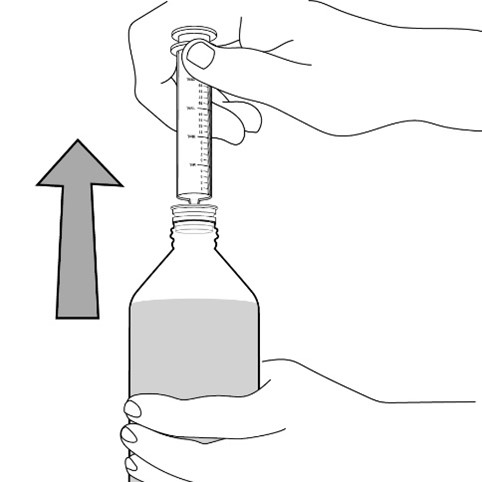
opening in the bottle adapter.
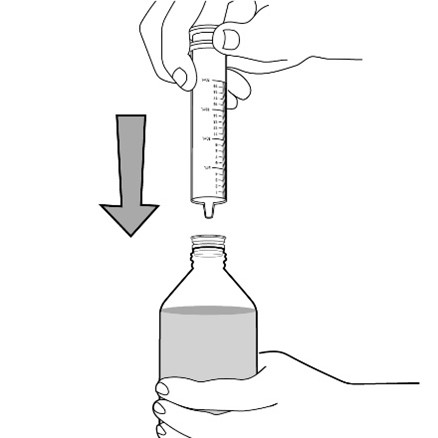
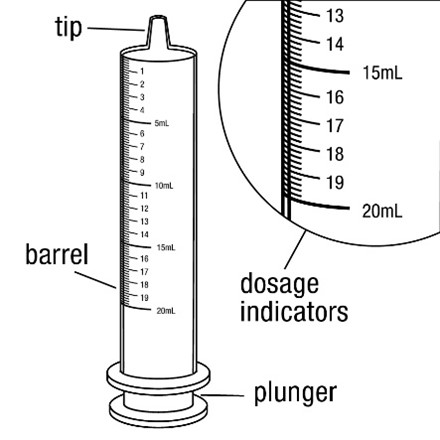
healthcare provider (the amount of liquid medicine in Step 4). If you see air bubbles in the oral syringe, fully push in the
plunger so that the oral solution flows back into the bottle. Then, withdraw the prescribed dose of oral suspension.
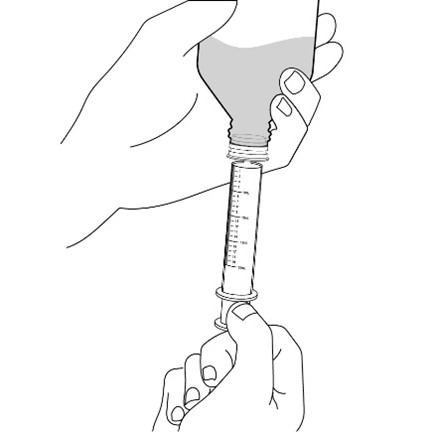
Measure the mLs of medicine from the end of the plunger.
• 2 syringes
• 1 syringe, taking 2 steps to draw up the medicine in that same syringe
If the dose is 24 mL, draw up 20 mL in the first syringe and the remaining 4 mL in the second syringe.
If the dose is 24 mL, draw up 20 mL in the single syringe and squirt the medicine into the mouth, then draw up the
remaining 4 mL in that same syringe.
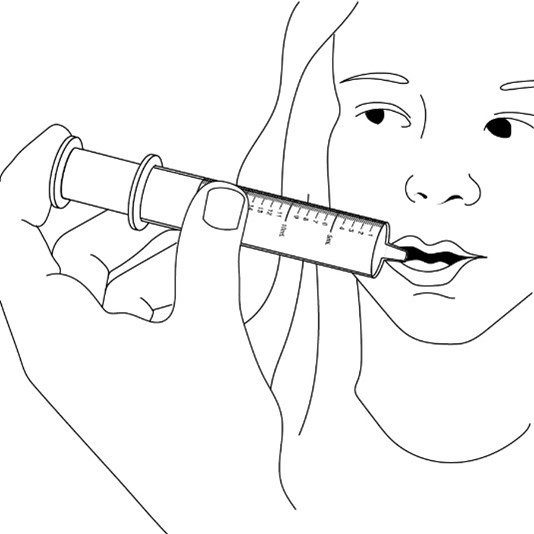
given. If you need 2 syringes for the dose, slowly squirt the medicine from the first syringe into the mouth, then slowly
squirt the medicine from the second syringe into the mouth.
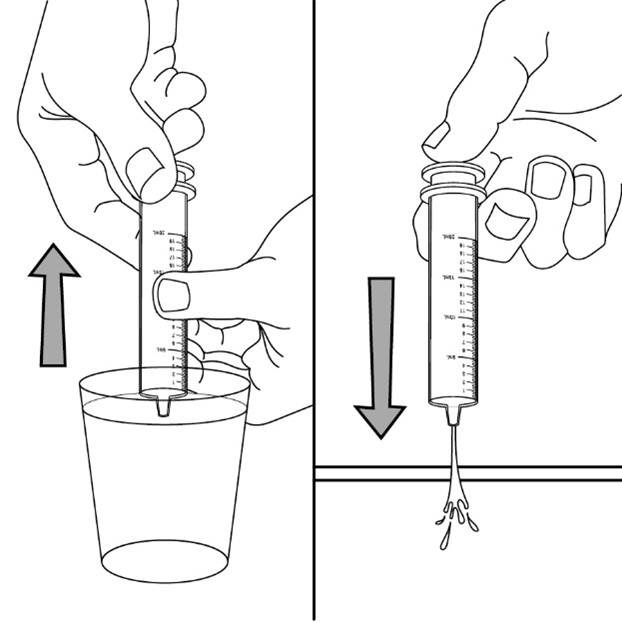
• Fill a cup with water
• Pull back on the plunger and draw the water from the cup into the syringe
• Push down on the plunger to release the water into the sink
- Store FYCOMPA oral suspension below 86°F (30°C). Do notfreeze.
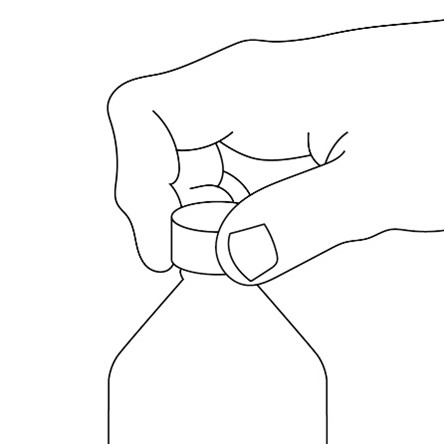
- Replace the cap tightly after opening.
- Use FYCOMPA oral suspension within 90 days after the bottle is first opened.
- After 90 days safely throw away any FYCOMPA Oral Suspension that has not been used.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
FYCOMPA® is a registered trademark owned by Catalyst Pharmaceuticals, Inc.
Marketed by Catalyst Pharmaceuticals, Inc., Coral Gables, FL 33134
©2023 Catalyst Pharmaceuticals, Inc.
Revised: 06/2023