Gallium Citrate Ga-67
Gallium Citrate Ga-67 Prescribing Information
Gallium Citrate Ga 67 Injection may be useful to demonstrate the presence and extent of Hodgkin's disease, lymphoma, and bronchogenic carcinoma. Positive gallium Ga-67 uptake in the absence of prior symptoms warrants follow-up as an indication of a potential disease state. Gallium Citrate Ga 67 Injection may be useful as an aid in detecting some acute inflammatory lesions.
The recommended adult (70 kg) dose of Gallium Citrate Ga 67 Injection is 74 to 185 megabecquerels (2 to 5 millicuries). Gallium Citrate Ga 67 Injection is intended for intravenous administration only.
Approximately 10 percent of the administered dose is excreted in the feces during the first week after injection. Daily laxatives and/or enemas are recommended from the day of injection until the final images are obtained in order to cleanse the bowel of radioactive material and minimize the possibility of false positive studies.
Studies indicate the optimal tumor to background concentration ratios are often obtained 48 hours post injection. However, considerable biological variability may occur in individuals and acceptable images may be obtained as early as 6 hours and as late as 120 hours after injection.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not use if contents are turbid.
Instructions for the handling of Gallium Citrate Ga 67:
- Waterproof gloves should be used during the entire handling and administration procedure.
- Using proper shielding, the vial containing the Gallium Citrate Ga 67 should be visually inspected to ensure that it is free of particulate matter and discoloration prior to use.
- Maintain adequate shielding during the life of the product and use a sterile, shielded syringe for withdrawing and injecting the preparation.
The estimated absorbed radiation doses
2from an intravenous injection of 185 megabecquerels (5 millicuries) of Gallium Citrate Ga 67 are shown in Table 4.
| Tissue | mGy/ 185MBq | rads/ 5mCi |
| Whole Body Skeleton Liver Bone Marrow Spleen Kidney Ovaries Testes Gastrointestinal Tract Stomach Small Intestine Upper Large Intestine Lower Large Intestine | 13.0 22.0 23.0 29.0 26.5 20.5 14.0 12.0 11.0 18.0 28.0 45.0 | 1.30 2.20 2.30 2.90 2.65 2.05 1.40 1.20 1.10 1.80 2.80 4.50 |
2 MIRD Dose Estimate Report No. 2, J. Nucl. Med. 14; 755-6 (1973).
None.
Rare occurrences of allergic reactions, skin rash and nausea have been reported in association with Gallium Citrate Ga 67 use.
Gallium Citrate Ga 67 Injection is supplied in a 10 milliliter vial as an isotonic, sterile, non-pyrogenic solution. Each milliliter of the isotonic solution contains 74 megabecquerels (2 millicuries) of gallium Ga-67 on the calibration date as a complex formed from 8.3 nanograms gallium chloride Ga-67, 1.9 milligrams of sodium citrate dihydrate, 7.8 milligrams of sodium chloride and 0.9 percent benzyl alcohol (v/v) as a preservative. The pH is adjusted to between 5.5 to 8.0 with hydrochloric acid and/or sodium hydroxide solution.
Gallium Ga-67, with a half-life of 78.26 hours, is cyclotron produced by the proton irradiation of enriched zinc. At the time of calibration the drug contains no more than 0.02% gallium Ga-66 and no more than 0.2% zinc Zn-65. The concentration of each radionuclidic impurity changes with time. At expiration, the drug contains no more than 0.001% gallium Ga-66 and no more than 1.0% zinc Zn-65. No carrier has been added.
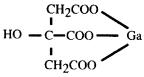
Gallium citrate has the following chemical structure:
Gallium Ga-67 with a physical half-life of 78.26 hours
1decays by electron capture to stable zinc Zn-67. Photons that are useful for imaging studies are listed in Table 1.
| Radiation | Mean Percent Per Disintegration | Energy (keV) |
| Gamma-2 Gamma-3 Gamma-4 Gamma-5 Gamma-6 Gamma-7 | 2.9 35.7 19.7 2.2 16.0 4.5 | 91.3 93.3 184.6 209.0 300.2 393.5 |
1 Kocher, D.C., Radioactive Decay Data Tables, Health and Safety Research Division, National Technical Information Service, DOE/TIC-11026, pg. 80, 1981.
The specific gamma ray constant for gallium Ga-67 is 1.6 R/mCi-hour at 1 cm. The first half-value thickness of lead (Pb) is 0.066 cm. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of lead is shown in Table 2. For example, the use of 1.2 cm of lead will decrease the radiation exposure by a factor of about 100.
| Shield Thickness (Pb), cm | Coefficient of Attenuation |
| 0.066 0.41 1.2 2.5 4.8 | 0.5 10 -1 10 -2 10 -3 10 -4 |
To correct for physical decay of this radionuclide, the fractions that remain at selected time intervals after the time of calibration are shown in Table 3.
| Hours | Fraction Remaining | Hours | Fraction Remaining |
| 0* 6 12 18 24 (1d) 30 36 42 48 (2d) 54 60 66 | 1.000 0.948 0.899 0.853 0.809 0.767 0.727 0.689 0.654 0.620 0.588 0.557 | 72 (3d) 78 84 90 96 (4d) 108 120 (5d) 132 144 (6d) 156 168 (7d) | 0.529 0.501 0.475 0.451 0.427 0.384 0.345 0.311 0.279 0.251 0.226 |
* Calibration Time
Gallium Citrate Ga 67, with no carrier added, has been found to concentrate in certain viable primary and metastatic tumors as well as focal sites of infection. The mechanism of concentration is unknown, but investigational studies have shown that gallium Ga-67 accumulates in lysosomes and is bound to a soluble intracellular protein.
It has been reported in the scientific literature that following intravenous injection, the highest tissue concentration of gallium Ga-67 - other than tumors and sites of infection - is the renal cortex. After the first day, the maximum concentration shifts to bone and lymph nodes and after the first week, to liver and spleen. Gallium Ga-67 is excreted relatively slowly from the body. The average whole body retention is 65 percent after seven days, with 26 percent having been excreted in the urine and 9 percent in the stools.