Gastrografin
(Diatrizoate Meglumine And Diatrizoate Sodium)Gastrografin Prescribing Information
Gastrografin (Diatrizoate Meglumine and
Diatrizoate Sodium Solution) is indicated for radiographic examination
of segments of the gastrointestinal tract (esophagus, stomach, proximal
small intestine, and colon). The preparation is particularly indicated
when a more viscous agent such as barium sulfate, which is not water-soluble,
is not feasible or is potentially dangerous.
Gastrografin may also be used as an adjunct to contrast
enhancement in computed tomography of the torso (body imaging); the
preparation is indicated, in conjunction with intravenous administration
of a radiopaque contrast agent, when unenhanced imaging may not provide
sufficient definition in distinguishing normal loops of bowel from
adjacent organs or areas of suspected pathology.
the preparation of solutions for parenteral administration. Oral or
rectal administration only. Discard any unused portion after procedure.
The routine preparatory measures
employed for barium studies are also appropriate for this agent.
For pediatric and severely cachectic patients
the maintenance of an intravenous fluid line may be advisable.
Do not administer to patients with a known
hypersensitivity to Gastrografin or any of its components.
Most adverse reactions to enteral diagnostic
radiopaque agents are mild and transitory. Nausea, vomiting and/or
diarrhea, urticaria with erythema, hypoxia, acute dyspnea, tachyarrhythmia,
and anaphylaxis have occurred following ingestion of the contrast
medium, particularly when high concentrations of large volumes of
solution are administered. Severe changes in serum osmolarity and
electrolyte concentrations may produce shock-like states (see
Dehydration: Administration of hypertonic
Gastrografin solutions may lead to hypovolemia and hypotension due
to fluid loss from the intestine. A 1 in 4.6 (1:4.6) dilution of Gastrografin
yields an approximately isotonic 16.5 percent diatrizoate salts solution;
less dilute solutions are hypertonic and may lead to intraluminal
movement of fluid with resulting hypovolemia. In young or debilitated
children and in elderly cachectic persons, the loss of plasma fluid
may be sufficient to cause a shock-like state. If Gastrografin is
used in infants and children (under 10 kg) or in dehydrated or debilitated
patients, the solution must be prepared using the specific dilutions
described in DOSAGE AND ADMINISTRATION. In
debilitated patients and in patients with electrolyte imbalances,
postprocedural monitoring of hydration, serum osmolarity, electrolytes
and clinical status is essential. In pediatric or severely debilitated
patients, the maintenance of an open intravenous fluid line for rehydration
may be advisable should hypotension or shock supervene. Electrolyte
disturbances must be corrected prior to the administration of any
hypertonic Gastrografin solutions.
Aspiration: Aspiration of Gastrografin into the trachea and airways
may result in serious pulmonary complications including, pulmonary
edema, pneumonitis or death Bronchial entry of any orally administered
contrast medium causes a copious osmotic effusion. Therefore, avoid
use of Gastrografin in patients with esophagotracheal fistula and
minimize risks for pulmonary aspiration in all patients. If Gastrografin
is given by nasogastric tube, the position of the tube in the stomach
must be verified before administration.
Anaphylactic reactions: Anaphylactic reactions, including
fatalities, have been reported with the use of Gastrografin. Patients
at increased risk include those with a history of a previous reaction
to a contrast medium, patients with a known sensitivity to iodine,
and patients with a known clinical hypersensitivity (bronchial asthma,
hay fever, and food allergies). Medical personnel trained in the treatment
of anaphylactic reactions and the necessary drugs and medical equipment
should always be readily available when Gastrografin is used.
that serious or anaphylactoid reactions that may occur with intravascular
administration of radiopaque contrast agents are theoretically possible
following administration by other routes.
Gastrografin (Diatrizoate Meglumine and Diatrizoate
Sodium Solution) is a palatable lemon-flavored water-soluble iodinated
radiopaque contrast medium for oral or rectal administration only.
Each mL contains 660 mg diatrizoate meglumine and 100 mg diatrizoate
sodium; pH has been adjusted to 6.0 to 7.6 with sodium hydroxide.
367 mg organically bound iodine. Inactive ingredients: edetate disodium,
flavor, polysorbate 80, purified water, saccharin sodium, simethicone,
and sodium citrate.
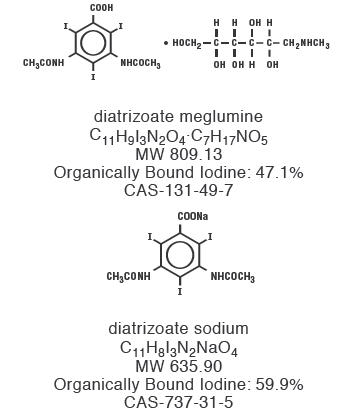
Diatrizoate
meglumine is designated chemically as 1-deoxy-1-(methylamino)-D-glucitol
3,5-diacetamido-2,4,6-triiodo-benzoate (salt); diatrizoate sodium
is monosodium 3, 5-diacetamido-2,4,6-triiodobenzoate. Structural formulas:
The most important characteristic of contrast media is the iodine
content. The relatively high atomic weight of iodine contributes sufficient
radiodensity for radiographic contrast with surrounding tissues.
Diagnostic enteral radiopaque agents have
few known pharmacological effects. Diatrizoate meglumine and diatrizoate
sodium exert a mild laxative effect attributable to their high osmolarity.
Diatrizoate meglumine and diatrizoate sodium
are sparingly absorbed from the intact gastrointestinal tract, and
therefore permit gastrointestinal opacification and delineation after
oral or rectal administration. Oral administration is used for radiographic
evaluation of the esophagus, stomach and proximal small intestine.
Rectal administration is used for examination of the colon; however,
visualization of the distal small bowel is generally unsatisfactory,
since the hypertonicity of the medium causes intraluminal diffusion
of water with subsequent dilution of the medium. Enough absorption
from the gastrointestinal tract to permit incidental visualization
of the urinary tract has been reported; this should also be considered
when thyroid testing is being contemplated, since iodine-mediated
thyrotropic effects may occur (see
Diagnostic procedures
which involve the use of radiopaque contrast agents should be carried
out under the direction of personnel with the prerequisite training
and with a thorough knowledge of the particular procedure to be performed.
Appropriate facilities should be available for coping with any complication
of administration, as well as for treatment of reaction to the contrast
medium (see
Rectal administration
of undiluted Gastrografin (Diatrizoate Meglumine and Diatrizoate Sodium
Solution) in any patient, particularly with large doses and/or in
those with overdistention, has been reported to be associated with
mucosal irritation.
Cases of hyperthyroidism
have been reported with the use of oral contrast media. Some of these
patients reportedly had multinodular goiters which may have been responsible
for the increased hormone synthesis in response to excess iodine.
Administration of an intravascular iodinated radiopaque diagnostic
agent to a hyperthyroid patient precipitated thyroid storm; a similar
situation could follow administration of oral preparations of iodides.
Therefore, caution should be exercised when administering enteral
gastrointestinal radiopaque agents to hyperthyroid and euthyroid goiterous
patients.
Consideration should
be given to the potential for precipitation of water-soluble contrast
agents under conditions that may promote hyperacidity (i.e., fasting,
emotional upset, or stress). Harmful effects directly attributable
to precipitate formation have not been reported. However, the possibility
of interpreting the precipitate radiologically as an anatomical abnormality
(i.e., ulceration of the stomach or small intestine) or injury, should
be kept in mind.
Patients should receive the following information and
instructions:
- This drug has been prescribed to perform an x-ray of the
gastrointestinal tract. - Inform the physician if pregnant or if allergic to iodine,
any foods, or x-ray materials. - The iodine in diatrizoate salts may interfere with some
thyroid tests if these are needed in the future. Inform the attending
physician at that time about this gastrointestinal study. - This drug may cause abdominal cramping, nausea, vomiting,
diarrhea, skin rashes, itching, heartburn, dizziness, or headache
in some patients, but most reactions are mild and pass quickly.
Tests
The results
of protein bound iodine (PBI) and radioactive iodine uptake studies,
which depend on iodine estimations, will not accurately reflect thyroid
function for six months, and possibly as long as one year, following
the administration of diagnostic enteral radiopaque media.
Thyroid function tests, if indicated, generally
should be performed prior to the administration of any iodinated agent.
However, thyroid function can be evaluated after use of these agents
by using T3resin uptake and total or free
thyroxine (T4) assays which are not dependent
on iodine estimations.
Small quantities of
contrast medium in the intestinal tract may cause false low trypsin
values when determined spectrophotometrically. Therefore, duodenal
instillation should not precede pancreatic function tests involving
spectrophotometric trypsin assays.
Any test which might be affected by contrast media should be performed
prior to administration of the contrast medium.
Impairment of Fertility
Long-term studies in animals have not been performed to evaluate
carcinogenic or mutagenic potential, or possible impairment of fertility
in males or females.
When administered
intravenously, diatrizoate salts cross the placenta and are evenly
distributed in fetal tissues.
No teratogenic effects attributable to diatrizoate meglumine or diatrizoate
sodium have been observed in teratology studies performed in animals.
There are, however, no adequate and well-controlled studies in pregnant
women. Because small amounts of these agents may be absorbed, and
animal teratology studies are not always predictive of human response,
these agents should be used during pregnancy only when clearly needed.
Procedures including radiation involve a
certain risk related to the exposure of the fetus.
Diatrizoate meglumine is excreted in breast
milk following intravascular administration.
Because small amounts of enteral gastrointestinal radiopaque
agents may be absorbed following oral or rectal administration, caution
should be exercised when they are administered to a nursing woman.
See
Local injury to the colonic
mucosa, particularly in the presence of underlying disease which interferes
with intestinal viability, has been reported in cases where recommended
doses and dilutions (see
polysorbate 80 level in the dose may be a contributing factor to injury.