Genosyl - Nitric Oxide gas
(Nitric Oxide)Genosyl - Nitric Oxide gas Prescribing Information
GENOSYL
®is indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents.
The recommended dose is 20 ppm, maintained for up to 14 days or until the underlying oxygen desaturation has resolved (
The recommended dose of GENOSYL is 20 ppm. Maintain treatment up to 14 days or until the underlying oxygen desaturation has resolved and the neonate is ready to be weaned from GENOSYL therapy.
Doses greater than 20 ppm are not recommended
Doses greater than 20 ppm are not recommended (
The recommended dose of GENOSYL is 20 ppm. Maintain treatment up to 14 days or until the underlying oxygen desaturation has resolved and the neonate is ready to be weaned from GENOSYL therapy.
Doses greater than 20 ppm are not recommended
Nitric oxide combines with hemoglobin to form methemoglobin, which does not transport oxygen. Methemoglobin levels increase with the dose of GENOSYL; it can take 8 hours or more before steady-state methemoglobin levels are attained. Monitor methemoglobin and adjust the dose of GENOSYL to optimize oxygenation.
If methemoglobin levels do not resolve with decrease in dose or discontinuation of GENOSYL, additional therapy may be warranted to treat methemoglobinemia
Administration: Avoid abrupt discontinuation (
GENOSYL must be administered using a calibrated GENOSYL Delivery System. Only validated ventilator systems should be used in conjunction with GENOSYL
Consult the GENOSYL Delivery System Operator's Manual or call 1-877-337-4118 or visit
Keep available a backup power supply to address power failures. The GENOSYL Delivery System consists of a primary system and a fully functional second system that can be used as backup in the event of primary system failure.
Measure methemoglobin within 4-8 hours after initiation of treatment with GENOSYL and periodically throughout treatment
Monitor for PaO2and inspired NO2during GENOSYL administration
The concentration of nitric oxide, nitrogen dioxide and air is constantly monitored. The GENOSYL Delivery System will shutdown if nitrogen dioxide reaches 3 ppm.
Avoid abrupt discontinuation of GENOSYL
Wean from GENOSYL
GENOSYL (nitric oxide) is a gas available at concentrations up to 800 ppm
GENOSYL (nitric oxide) is administered by inhalation. Nitric oxide is a pulmonary vasodilator. Nitric oxide is generated from liquid dinitrogen tetroxide (N2O4) by the cassette in the GENOSYL Delivery System. Upon initiation of GENOSYL Delivery System, the liquid N2O4is heated and the equilibrium shifts to nitrogen dioxide (NO2) gas. The NO2is then converted into nitric oxide (NO) using the antioxidant cartridges, and nitric oxide is delivered to the patient by means of a ventilator or a nasal cannula. The amount of nitric oxide administered to the patient is set by controlling the temperature of the N2O4liquid module, which controls the pressure inside the liquid module, which in turn controls the mass of NO2that is sent to the primary cartridges, and hence the mass of nitric oxide. The mass flow of nitric oxide, together with the air from the pump, control the nitric oxide concentration. A nitric oxide sensor monitors the nitric oxide in the patient line. GENOSYL Delivery System is designed to deliver a controlled level of nitric oxide blended with breathing air or oxygen-enriched breathing air.
The GENOSYL Delivery System controls the flow of nitric oxide mixed with air delivered to the patient.
The structural formula of nitric oxide (NO) is shown below:
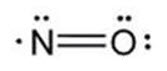
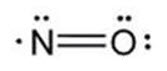
The safety and efficacy of nitric oxide for inhalation has been demonstrated in term and near-term neonates with hypoxic respiratory failure associated with evidence of pulmonary hypertension
The efficacy of nitric oxide gas was investigated in term and near-term newborns with hypoxic respiratory failure (HRF) resulting from a variety of etiologies. Inhalation of nitric oxide gas reduces the oxygenation index (OI= mean airway pressure in cm H2O × fraction of inspired oxygen concentration [FiO2]× 100 divided by systemic arterial concentration in mm Hg [PaO2]) and increases PaO2
The Neonatal Inhaled Nitric Oxide Study (NINOS) was a double-blind, randomized, placebo-controlled, multicenter trial in 235 neonates with hypoxic respiratory failure. The objective of the study was to determine whether inhaled nitric oxide would reduce the occurrence of death and/or initiation of extracorporeal membrane oxygenation (ECMO) in a prospectively defined cohort of term or near-term neonates with hypoxic respiratory failure unresponsive to conventional therapy. Hypoxic respiratory failure was caused by meconium aspiration syndrome (MAS; 49%), pneumonia/sepsis (21%), idiopathic primary pulmonary hypertension of the newborn (PPHN; 17%), or respiratory distress syndrome (RDS; 11%). Infants 14 days of age (mean, 1.7 days) with a mean PaO2of 46 mm Hg and a mean oxygenation index (OI) of 43 cm H2O / mm Hg were initially randomized to receive 100% O2with (n=114) or without (n=121) 20 ppm nitric oxide for up to 14 days. Response to study drug was defined as a change from baseline in PaO230 minutes after starting treatment (full response = >20 mm Hg, partial = 10-20 mm Hg, no response = <10 mm Hg). Neonates with a less than full response were evaluated for a response to 80 ppm nitric oxide or control gas. The primary results of this study are presented in Table 1.
| Control (n=121) | Nitric Oxide gas (n=114) | P value | |
|---|---|---|---|
| Death or ECMODeath or need for ECMO was the primary end point of this studyExtracorporeal membrane oxygenation | 77 (64%) | 52 (46%) | 0.006 |
| Death | 20 (17%) | 16 (14%) | 0.60 |
| ECMO | 66 (55%) | 44 (39%) | 0.014 |
Although the incidence of death by 120 days of age was similar in both groups (NO, 14%; control 17%), significantly fewer infants in the nitric oxide group required ECMO compared with controls (39% vs. 55%, p = 0.014). The combined incidence of death and/or initiation of ECMO showed a significant advantage for the nitric oxide treated group (46% vs. 64%, p = 0.006). The nitric oxide group also had significantly greater increases in PaO2and greater decreases in the OI and the alveolar-arterial oxygen gradient than the control group (p<0.001 for all parameters). Significantly more patients had at least a partial response to the initial administration of study drug in the nitric oxide group (66%) than the control group (26%, p<0.001). Of the 125 infants who did not respond to 20 ppm nitric oxide control, similar percentages of NO-treated (18%) and control (20%) patients had at least a partial response to 80 ppm nitric oxide gas for inhalation or control drug, suggesting a lack of additional benefit for the higher dose of nitric oxide. No infant had study drug discontinued for toxicity. Inhaled nitric oxide gas had no detectable effect on mortality. The adverse events collected in the NINOS trial occurred at similar incidence rates in both treatment groups
This study was a double-blind, randomized, placebo-controlled, multi-center trial of 186 term and near-term neonates with pulmonary hypertension and hypoxic respiratory failure. The primary objective of the study was to determine whether nitric oxide gas would reduce the receipt of ECMO in these patients. Hypoxic respiratory failure was caused by MAS (35%), idiopathic PPHN (30%), pneumonia/sepsis (24%), or RDS (8%). Patients with a mean PaO2of 54 mm Hg and a mean OI of 44 cm H2O / mm Hg were randomly assigned to receive either 20 ppm nitric oxide gas (n=97) or nitrogen gas (placebo; n=89) in addition to their ventilatory support. Patients who exhibited a PaO2>60 mm Hg and a pH < 7.55 were weaned to 5 ppm nitric oxide gas or placebo. The primary results from the CINRGI study are presented in Table 2.
| Placebo | Nitric oxide gas | P value | |
|---|---|---|---|
| ECMOECMO was the primary end point of this studyExtracorporeal membrane oxygenation | 51/89 (57%) | 30/97 (31%) | <0.001 |
| Death | 5/89 (6%) | 3/97 (3%) | 0.48 |
Significantly fewer neonates in the nitric oxide gas group required ECMO compared to the control group (31% vs. 57%, p<0.001). While the number of deaths were similar in both groups (Nitric oxide gas, 3%; placebo, 6%), the combined incidence of death and/or receipt of ECMO was decreased in the nitric oxide gas group (33% vs. 58%, p<0.001).
In addition, the nitric oxide gas group had significantly improved oxygenation as measured by PaO2, OI, and alveolar-arterial gradient (p<0.001 for all parameters). Of the 97 patients treated with nitric oxide gas, 2 (2%) were withdrawn from study drug due to methemoglobin levels >4%. The frequency and number of adverse events reported were similar in the two study groups
In clinical trials, reduction in the need for ECMO has not been demonstrated with the use of inhaled nitric oxide in neonates with congenital diaphragmatic hernia (CDH).
The safety and efficacy of nitric oxide gas for the prevention of chronic lung disease [bronchopulmonary dysplasia (BPD)] in neonates ≤ 34 weeks gestational age requiring respiratory support has been studied in four large previously conducted multicenter, double-blind, placebo-controlled clinical trials in a total of 2,600 preterm infants. Of these, 1,290 received placebo, and 1,310 received inhaled nitric oxide at doses ranging from 5-20 ppm, for treatment periods of 7-24 days duration. The primary endpoint for these studies was alive and without BPD at 36 weeks postmenstrual age (PMA). The need for supplemental oxygen at 36 weeks PMA served as a surrogate endpoint for the presence of BPD. Overall, efficacy for the prevention of bronchopulmonary dysplasia in preterm infants was not established. There were no meaningful differences between treatment groups with regard to overall deaths, methemoglobin levels, or adverse events commonly observed in premature infants, including intraventricular hemorrhage, patent ductus arteriosus, pulmonary hemorrhage, and retinopathy of prematurity.
The use of GENOSYL (nitric oxide) for prevention of BPD in preterm neonates ≤34 weeks gestational age is not recommended.
GENOSYL is contraindicated in neonates dependent on right-to-left shunting of blood.
Rebound Pulmonary Hypertension: Abrupt discontinuation of GENOSYL may lead to worsening oxygenation and increasing pulmonary artery pressure (
Wean from GENOSYL
Methemoglobinemia: Methemoglobin increases with the dose of nitric oxide; following discontinuation or reduction of nitric oxide, methemoglobin levels return to baseline over a period of hours (
Nitric oxide combines with hemoglobin to form methemoglobin, which does not transport oxygen. Methemoglobin levels increase with the dose of GENOSYL; it can take 8 hours or more before steady-state methemoglobin levels are attained. Monitor methemoglobin and adjust the dose of GENOSYL to optimize oxygenation.
If methemoglobin levels do not resolve with decrease in dose or discontinuation of GENOSYL, additional therapy may be warranted to treat methemoglobinemia
Elevated NO
2Levels: Monitor NO
2levels (
Nitrogen dioxide (NO2) forms in gas mixtures containing NO and O2. Nitrogen dioxide may cause airway inflammation and damage to lung tissues.
If there is an unexpected change in NO2concentration, or if the NO2concentration reaches 0.5 ppm when measured in the breathing circuit, then the delivery system should be assessed in accordance with the GENOSYL Delivery System Operator's Manual troubleshooting section, and the NO2analyzer should be recalibrated. The dose of GENOSYL and/or FiO2should be adjusted as appropriate.
Heart Failure: In patients with pre-existing left ventricular dysfunction, GENOSYL may increase pulmonary capillary wedge pressure leading to pulmonary edema (
Patients with left ventricular dysfunction treated with GENOSYL may experience pulmonary edema, increased pulmonary capillary wedge pressure, worsening of left ventricular dysfunction, systemic hypotension, bradycardia and cardiac arrest. Discontinue GENOSYL while providing symptomatic care.