Glipizide
Glipizide Prescribing Information
Glipizide tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
There is no fixed dosage regimen for the management of diabetes mellitus with glipizide or any other hypoglycemic agent. In addition to the usual monitoring of urinary glucose, the patient's blood glucose must also be monitored periodically to determine the minimum effective dose for the patient; to detect primary failure, i.e., inadequate lowering of blood glucose at the maximum recommended dose of medication; and to detect secondary failure, i.e., loss of an adequate blood-glucose-lowering response after an initial period of effectiveness. Glycosylated hemoglobin levels may also be of value in monitoring the patient's response to therapy.
Short-term administration of glipizide tablets may be sufficient during periods of transient loss of control in patients usually controlled well on diet.
In general, glipizide tablets should be given approximately 30 minutes before a meal to achieve the greatest reduction in postprandial hyperglycemia.
Glipizide tablets are contraindicated in patients with:
- Known hypersensitivity to the drug.
- Type 1 diabetes mellitus, diabetic ketoacidosis, with or without coma. This condition should be treated with insulin.
In U.S. and foreign controlled studies, the frequency of serious adverse reactions reported was very low. Of 702 patients, 11.8% reported adverse reactions and in only 1.5% was glipizide discontinued.
Certain drugs tend to produce hyperglycemia and may lead to loss of control. These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blocking drugs, and isoniazid. When such drugs are administered to a patient receiving glipizide, the patient should be closely observed for loss of control. When such drugs are withdrawn from a patient receiving glipizide, the patient should be observed closely for hypoglycemia.
A potential interaction between oral miconazole and oral hypoglycemic agents leading to severe hypoglycemia has been reported. Whether this interaction also occurs with the intravenous, topical, or vaginal preparations of miconazole is not known. The effect of concomitant administration of DIFLUCAN
®(fluconazole) and glipizide has been demonstrated in a placebo-controlled crossover study in normal volunteers. All subjects received glipizide alone and following treatment with 100 mg of DIFLUCAN as a single daily oral dose for 7 days. The mean percentage increase in the glipizide AUC after fluconazole administration was 56.9% (range: 35 to 81).
In studies assessing the effect of colesevelam on the pharmacokinetics of glipizide ER in healthy volunteers, reductions in glipizide AUC
0–∞and C
maxof 12% and 13%, respectively were observed when colesevelam was coadministered with glipizide ER. When glipizide ER was administered 4 hours prior to colesevelam, there was no significant change in glipizide AUC
0–∞or C
max,-4% and 0%, respectively. Therefore, glipizide should be administered at least 4 hours prior to colesevelam to ensure that colesevelam does not reduce the absorption of glipizide.
Glipizide is an oral blood-glucose-lowering drug of the sulfonylurea class.
The Chemical Abstracts name of glipizide is 1-cyclohexyl-3-[[p-[2-(5-methylpyrazine-carboxamido)ethyl]phenyl]sulfonyl]urea. The molecular formula is C
21H
27N
5O
4S; the molecular weight is 445.55; the structural formula is shown below:
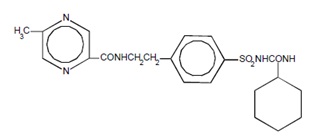
Glipizide is a whitish, odorless powder with a pKa of 5.9. It is insoluble in water and alcohols, but soluble in 0.1
Inactive ingredients are: colloidal silicon dioxide; croscarmellose sodium, lactose anhydrous; microcrystalline cellulose and stearic acid.
Meets USP Dissolution test 2.