Grafapex Prescribing Information
- GRAFAPEX causes severe and prolonged myelosuppression at the recommended dosage.
- Hematopoietic stem cell transplantation is required to prevent potentially fatal complications of the prolonged myelosuppression. Monitor hematologic laboratory parameters[see Warnings and Precautions (.)]
5.1 MyelosuppressionProfound myelosuppression with pancytopenia is the desired therapeutic effect of GRAFAPEX-based preparative regimens, occurring in all patients. Time to neutrophil counts > 0.5 Gi/L occurred at a median of 18 days (range 7-42 days) after allogeneic hematopoietic stem cell transplantation in adult patients treated using GRAFAPEX in combination with fludarabine as the preparative regimen.
Do not begin the preparative regimen if the stem cell donor is not available. Monitor blood cell counts daily until hematopoetic recovery. Provide standard supportive care for infections, anemia and thrombocytopenia until there is adequate hematopoietic recovery.
For injection: 1 g/vial or 5 g/vial treosulfan as a white, sterile, lyophilized powder in single-dose vials for reconstitution.
GRAFAPEX is contraindicated in patients with hypersensitivity to any component of the drug product.
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Myelosuppression[see Warnings and Precautions ()]
5.1 MyelosuppressionProfound myelosuppression with pancytopenia is the desired therapeutic effect of GRAFAPEX-based preparative regimens, occurring in all patients. Time to neutrophil counts > 0.5 Gi/L occurred at a median of 18 days (range 7-42 days) after allogeneic hematopoietic stem cell transplantation in adult patients treated using GRAFAPEX in combination with fludarabine as the preparative regimen.
Do not begin the preparative regimen if the stem cell donor is not available. Monitor blood cell counts daily until hematopoetic recovery. Provide standard supportive care for infections, anemia and thrombocytopenia until there is adequate hematopoietic recovery.
- Seizures[see Warnings and Precautions ()]
5.2 SeizuresThere have been reports of seizures in patients following treatment with treosulfan. Monitor patients for signs of neurological adverse reactions. Clonazepam prophylaxis may be considered for patients at higher risk for seizures, including infants.
- Skin Disorders[see Warnings and Precautions ()]
5.3 Skin DisordersAn increase of skin disorders (e.g. rash, dermatitis) was observed when patients received sodium bicarbonate-containing hydration in the course of treosulfan infusion, potentially because of acceleration of the pH‑dependent formation of alkylating epoxides
[see Adverse Reactions and Clinical Pharmacology ].Keep skin clean and dry on days of GRAFAPEX infusion. Diaper dermatitis may occur because of excretion of treosulfan in the urine. Change diapers frequently during the 12 hours after each infusion of GRAFAPEX. Dermatitis may occur under occlusive dressings; change occlusive dressings after each infusion of GRAFAPEX. - Injection Site Reactions and Tissue Necrosis[see Warnings and Precautions ()]
5.4 Injection Site Reactions and Tissue NecrosisGRAFAPEX may cause local tissue necrosis and injection site reactions, including erythema, pain, and swelling, in case of extravasation. Assure venous access patency prior to starting GRAFAPEX infusion, and monitor the intravenous infusion site for redness, swelling, pain, infection, and necrosis during and after administration of GRAFAPEX. If extravasation occurs, stop the infusion immediately and manage medically as required. Do not administer by the intramuscular or subcutaneous routes.
- Secondary Malignancies[see Warnings and Precautions ()]
5.5 Secondary MalignanciesThere is an increased risk of a secondary malignancy with use of GRAFAPEX. Treosulfan is carcinogenic and genotoxic
[see Nonclinical Toxicology ].The risk of secondary malignancy is increased in patients with Fanconi anemia and other DNA breakage disorders. The safety and efficacy of GRAFAPEX have not been established for patients with these disorders.
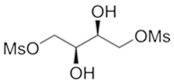
GRAFAPEX for injection contains treosulfan, an alkylating drug. Treosulfan is known chemically as L-‑threitol ‑1,4-‑dimethanesulfonate. Treosulfan is soluble in water (7% m/v) at 25ᴼC. Treosulfan is not hygroscopic.Treosulfan has the molecular formula C
6H
14O
8S
2and a molecular weight of 278.3 g/mole. Treosulfan has the following chemical structure:
GRAFAPEX is intended for intravenous administration. It is supplied as a white, sterile, lyophilized powder for injection in glass vials containing 1 g or 5 g treosulfan.
The efficacy of GRAFAPEX was evaluated in a randomized active-controlled trial (MC‑FludT.14/L Trial II;
NCT00822393) comparing GRAFAPEX to busulfan in combination with fludarabine as a preparative regimen for allogeneic transplantation. Eligible patients included adults 18 to 70 years old with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS), Karnofsky performance status > 60%, and age ≥ 50 years or hematopoietic cell transplantation comorbidity index [HCT‑CI] score > 2. Patients were excluded if the creatinine clearance was < 60 mL/min, forced expiratory volume (FEV1) < 50% or on supplemental oxygen, left ventricular ejection fraction (LVEF) < 40%, bilirubin > 3X ULN, or aminotransferases (ALT or AST) > 5X ULN.
The patients were randomized to receive GRAFAPEX 10 g/m² daily on day -4, -3 and -2 or to busulfan 0.8 mg/kg every 6 hours on day -4 and -3 in combination with fludarabine 30 mg/m
2daily on day -6, -5, -4, -3 and -2, and hematopoietic stem cell transplantation on day 0. For the subset of patients with unrelated donors, antithymocyte globulin was administered in 97% and 95% of patients on each arm, respectively. Cyclosporine and methotrexate was used as graft‑vs‑host disease prophylaxis.
There were 570 patients randomized to GRAFAPEX (n = 280) or busulfan (n = 290). The efficacy population included 365 patients with AML and 205 patients with MDS: 536 patients received peripheral blood stem cells, 15 patients received marrow stem cells, and 19 patients were not transplanted. Table 5 shows the baseline characteristics of the study patients.
Baseline Characteristics | GRAFAPEX (n = 280) | Busulfan (n = 290) |
|---|---|---|
Age, median (range), years | 60.0 (37, 70) | 60.5 (31, 70) |
Age, n (%)
|
76 (27%) | 218 (75%) 72 (25%) |
Gender, n (%)
| 171 (61%) | 176 (61%) |
Weight, median (range), kg | 80.0 (48.0, 144.0) | 78.1 (46.0, 141.9) |
Disease, n (%) AML MDS | 192 (69%) 88 (31%) | 173 (60%) 117 (40%) |
AML remission status, n (%)
| 164 (85%) 28 (15%) | 148 (86%) 25 (14%) |
MDS risk group, n (%)
| 20 (23%) 68 (77%) | 20 (17%) 97 (83%) |
HCT‑CI score
| 116 (41%) 164 (59%) | 118 (41%) 172 (59%) |
Donor
| 66 (24%) 214 (76%) | 68 (23%) 222 (77%) |
Abbreviations: AML: acute myeloid leukemia; MDS: myelodysplastic syndrome; CR1: complete remission 1; HCT-CI: hematopoietic cell transplant-specific comorbidity index | ||
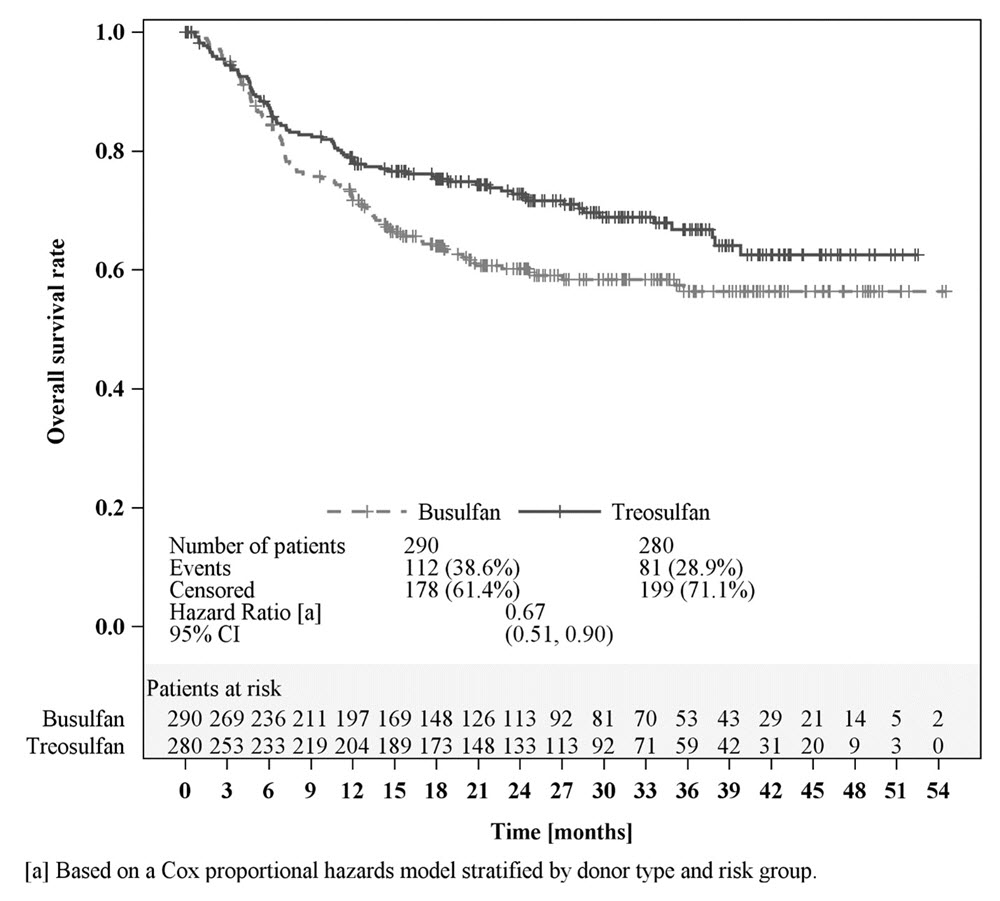
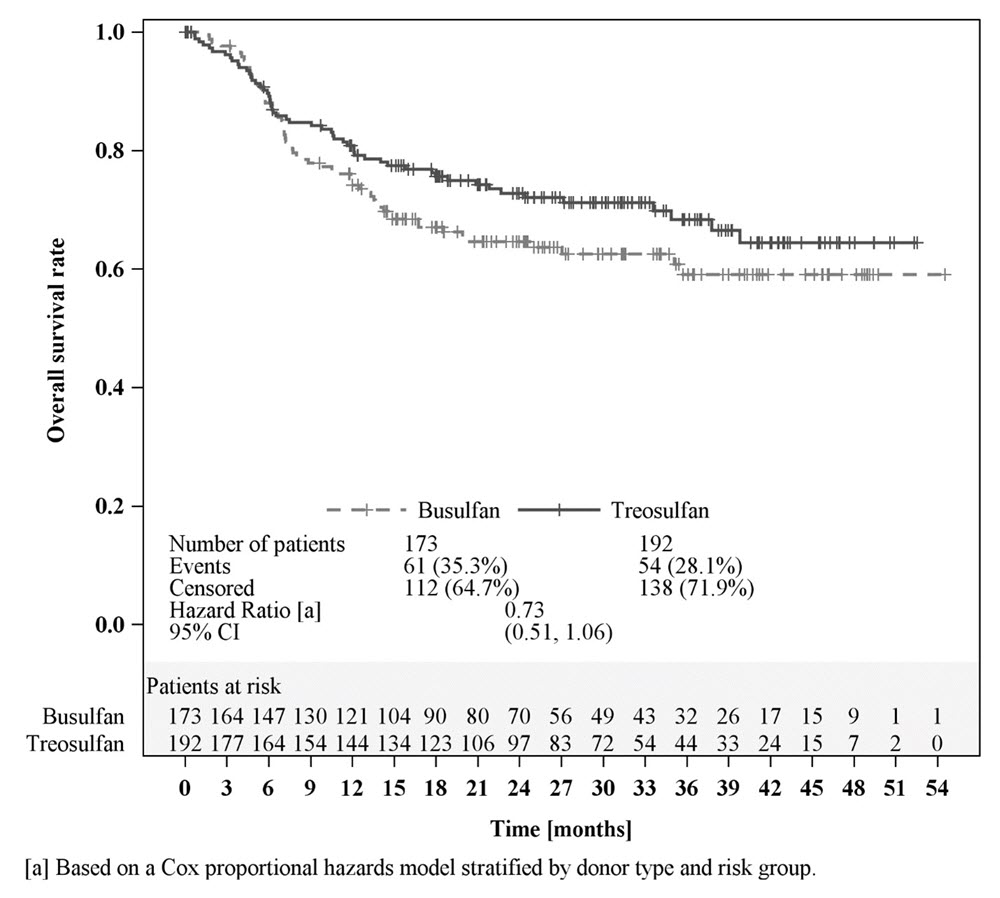
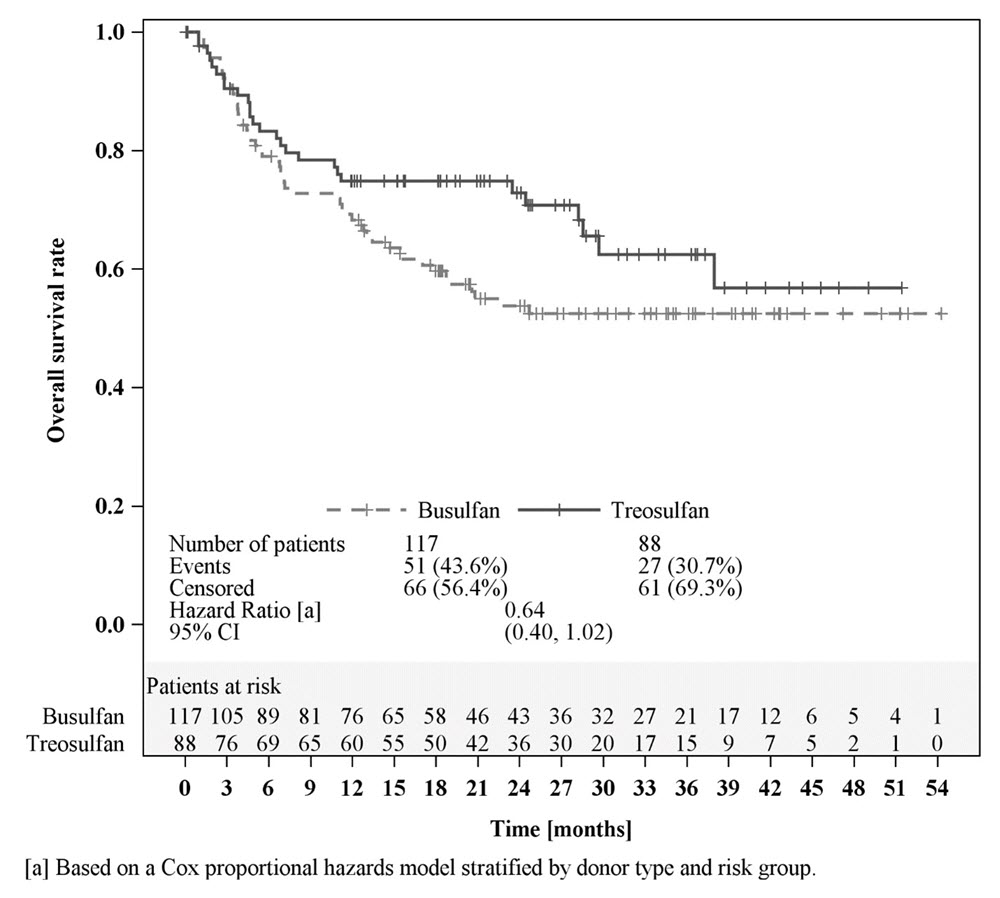
Efficacy was established on the basis of overall survival (OS), defined as the time from randomization until death from any cause. The hazard ratio (HR) for OS (stratified by donor type and risk group) compared to busulfan was 0.67 (95% CI: 0.51, 0.90) in the randomized population, 0.73 (95% CI: 0.51, 1.06) in patients with AML, and 0.64 (95% CI: 0.40, 1.02) in patients with MDS. Results are displayed in Figures 1, 2, and 3 below.