Guanfacine
Guanfacine Prescribing Information
Guanfacine tablets are indicated in the management of hypertension. Guanfacine tablets may be given alone or in combination with other antihypertensive agents, especially thiazide-type diuretics.
The recommended initial dose of guanfacine tablets, USP when given alone or in combination with another antihypertensive drug is 1 mg daily given at bedtime to minimize somnolence. If after 3 to 4 weeks of therapy 1 mg does not give a satisfactory result, a dose of 2 mg may be given, although most of the effect of guanfacine hydrochloride is seen at 1 mg (see
The frequency of rebound hypertension is low, but it can occur. When rebound occurs, it does so after 2 - 4 days, which is delayed compared with clonidine hydrochloride. This is consistent with the longer half-life of guanfacine. In most cases, after abrupt withdrawal of guanfacine, blood pressure returns to pretreatment levels slowly (within 2 - 4 days) without ill effects.
Guanfacine tablets are contraindicated in patients with known hypersensitivity to guanfacine hydrochloride.
Adverse reactions noted with guanfacine hydrochloride are similar to those of other drugs of the central α
2-adrenoreceptor agonist class: dry mouth, sedation (somnolence), weakness (asthenia), dizziness, constipation, and impotence. While the reactions are common, most are mild and tend to disappear on continued dosing.
Skin rash with exfoliation has been reported in a few cases; although clear cause and effect relationships to guanfacine hydrochloride could not be established, should a rash occur, guanfacine hydrochloride should be discontinued and the patient monitored appropriately.
In the dose-response monotherapy study described under
Adverse Reaction | Placebo n=59 | 0.5 mg n=60 | 1 mg n=61 | 2 mg n=60 | 3 mg n=59 |
| Dry Mouth | 0% | 10% | 10% | 42% | 54% |
| Somnolence | 8% | 5% | 10% | 13% | 39% |
| Asthenia | 0% | 2% | 3% | 7% | 3% |
| Dizziness | 8% | 12% | 2% | 8% | 15% |
| Headache | 8% | 13% | 7% | 5% | 3% |
| Impotence | 0% | 0% | 0% | 7% | 3% |
| Constipation | 0% | 2% | 0% | 5% | 15% |
| Fatigue | 2% | 2% | 5% | 8% | 10% |
The percent of patients who dropped out because of adverse reactions are shown below for each dosage group.
Placebo | 0.5 mg | 1 mg | 2 mg | 3 mg | |
| Percent dropouts | 0% | 2.0% | 5.0% | 13% | 32% |
The most common reasons for dropouts among patients who received guanfacine were dry mouth, somnolence, dizziness, fatigue, weakness, and constipation.
In the 12-week, placebo-controlled, dose-response study of guanfacine administered with 25 mg chlorthalidone at bedtime, the frequency of the most commonly observed adverse reactions showed a clear dose relationship from 0.5 to 3 mg as follows:
Adverse Reaction | Placebo n=73 | 0.5 mg n=72 | 1 mg n=72 | 2 mg n=72 | 3 mg n=72 |
| Dry Mouth | 5 (7%) | 4 (5%) | 6 (8%) | 8 (11%) | 20 (28%) |
| Somnolence | 1 (1%) | 3 (4%) | 0 (0%) | 1 (1%) | 10 (14%) |
| Asthenia | 0 (0%) | 2 (3%) | 0 (0%) | 2 (2%) | 7 (10%) |
| Dizziness | 2 (2%) | 1 (1%) | 3 (4%) | 6 (8%) | 3 (4%) |
| Headache | 3 (4%) | 4 (3%) | 3 (4%) | 1 (1%) | 2 (2%) |
| Impotence | 1 (1%) | 1 (0%) | 0 (0%) | 1 (1%) | 3 (4%) |
| Constipation | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 1 (1%) |
| Fatigue | 3 (3%) | 2 (3%) | 2 (3%) | 5 (6%) | 3 (4%) |
There were 41 premature terminations because of adverse reactions in this study. The percent of patients who dropped out and the dose at which the dropout occurred were as follows:
Dose | Placebo | 0.5 mg | 1 mg | 2 mg | 3 mg |
| Percent dropouts | 6.9% | 4.2% | 3.2% | 6.9% | 8.3% |
Reasons for dropouts among patients who received guanfacine were: somnolence, headache, weakness, dry mouth, dizziness, impotence, insomnia, constipation, syncope, urinary incontinence, conjunctivitis, paresthesia, and dermatitis.
In a second 12-week placebo-controlled combination therapy study in which the dose could be adjusted upward to 3 mg per day in 1-mg increments at 3-week intervals, i.e., a setting more similar to ordinary clinical use, the most commonly recorded reactions were: dry mouth, 47%; constipation, 16%; fatigue, 12%; somnolence, 10%; asthenia, 6%; dizziness, 6%; headache, 4%; and insomnia, 4%.
Reasons for dropouts among patients who received guanfacine were: somnolence, dry mouth, dizziness, impotence, constipation, confusion, depression, and palpitations.
In the clonidine/guanfacine comparison described in
Adverse Reactions | Guanfacine (n=279) | Clonidine (n=278) |
| Dry Mouth | 30% | 37% |
| Somnolence | 21% | 35% |
| Dizziness | 11% | 8% |
| Constipation | 10% | 5% |
| Fatigue | 9% | 8% |
| Headache | 4% | 4% |
| Insomnia | 4% | 3% |
Adverse reactions occurring in 3% or less of patients in the three controlled trials of guanfacine hydrochloride with a diuretic were:
Cardiovascular - bradycardia, palpitations, substernal pain Gastrointestinal - abdominal pain, diarrhea, dyspepsia, dysphagia, nausea CNS - amnesia, confusion, depression, insomnia, libido decrease
ENT disorders - rhinitis, taste perversion, tinnitus
Eye disorders - conjunctivitis, iritis, vision disturbance Musculoskeletal - leg cramps, hypokinesia
Respiratory - dyspnea
Dermatologic - dermatitis, pruritus, purpura, sweating
Urogenital - testicular disorder, urinary incontinence
Other - malaise, paresthesia, paresis
Adverse reaction reports tend to decrease over time. In an open-label trial of one year's duration, 580 hypertensive subjects were given guanfacine, titrated to achieve goal blood pressure, alone (51%), with diuretic (38%), with beta blocker (3%), with diuretic plus beta blocker (6%), or with diuretic plus vasodilator (2%). The mean daily dose of guanfacine reached was 4.7 mg.
Adverse Reaction | Incidence of adverse reactions at any time during the study n = 580 | Incidence of adverse reactions at end of one year n = 580 |
| Dry Mouth | 60% | 15% |
| Drowsiness | 33% | 6% |
| Dizziness | 15% | 1% |
| Constipation | 14% | 3% |
| Weakness | 5% | 1% |
| Headache | 4% | 0.2% |
| Insomnia | 5% | 0% |
There were 52 (8.9%) dropouts due to adverse effects in this 1-year trial. The causes were: dry mouth (n = 20), weakness (n = 12), constipation (n = 7), somnolence (n = 3), nausea (n = 3), orthostatic hypotension (n = 2), insomnia (n = 1), rash (n = 1), nightmares (n = 1), headache (n = 1), and depression (n = 1).
The potential for increased sedation when guanfacine hydrochloride is given with other CNS-depressant drugs should be appreciated.
The administration of guanfacine concomitantly with a known microsomal enzyme inducer (phenobarbital or phenytoin) to two patients with renal impairment reportedly resulted in significant reductions in elimination half-life and plasma concentration. In such cases, therefore, more frequent dosing may be required to achieve or maintain the desired hypotensive response. Further, if guanfacine is to be discontinued in such patients, careful tapering of the dosage may be necessary in order to avoid rebound phenomena (see
Guanfacine Tablets, USP are centrally acting antihypertensive with α
2-adrenoceptor agonist properties in tablet form for oral administration.
The chemical name of guanfacine hydrochloride is N-amidino-2-(2,6-dichlorophenyl) acetamide hydrochloride and its molecular weight is 282.56. Its structural formula is:
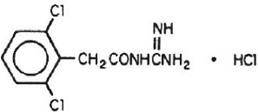
Guanfacine hydrochloride is a white to off-white powder; sparingly soluble in water and alcohol and slightly soluble in acetone. The inactive ingredients in each tablet are crospovidone, fumaric acid, lactose monohydrate, magnesium stearate, pregelatinized maize starch. Additionally 1 mg tablet contains FD & C blue 1 Alu lake.
FDA approved dissolution test specifications differ from USP.