Guanfacine
Guanfacine Prescribing Information
Guanfacine extended-release tablets are indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) as monotherapy and as adjunctive therapy to stimulant medications
Efficacy of guanfacine extended-release tablets in the treatment of ADHD was established in children and adolescents (6 to 17 years) in:
• Five short-term, placebo-controlled monotherapy trials (Studies 1, 2, 4, 5, and 6).
• One short-term, placebo-controlled adjunctive trial with psychostimulants (Study 3).
• One long-term, placebo-controlled monotherapy maintenance trial (Study 7).
Study 1 (301 study) was a double-blind, placebo-controlled, parallel-group, fixed-dose study, in which efficacy of once daily dosing with guanfacine extended-release tablets (2 mg, 3 mg and 4 mg) was evaluated for 5 weeks (n=345) in children and adolescents aged 6-17 years. Study 2 (304 study) was a double-blind, placebo-controlled, parallel-group, fixed-dose study, in which efficacy of once daily dosing with guanfacine extended-release tablets (1 mg, 2 mg, 3 mg and 4 mg) was evaluated for 6 weeks (n=324) in children and adolescents aged 6-17 years. In both studies, randomized patients in 2 mg, 3 mg and 4 mg dose groups were titrated to their target fixed dose, and continued on the same dose until a dose tapering phase started. The lowest dose of 1 mg used in Study 2 was not randomized to patients weighing more than 50 kg. Patients who weighed less than 25 kg were not included in either study.
Signs and symptoms of ADHD were evaluated on a once weekly basis using the clinician administered and scored ADHD Rating Scale (ADHD-RS-IV), which includes both hyperactive/impulsive and inattentive subscales. The primary efficacy outcome was the change from baseline to endpoint in ADHD-RS-IV total scores. Endpoint was defined as the last post-randomization treatment week for which a valid score was obtained prior to dose tapering (up to Week 5 in Study 1 and up to Week 6 in Study 2).
The mean reductions in ADHD-RS-IV total scores at endpoint were statistically significantly greater for guanfacine extended-release tablets compared to placebo for Studies 1 and 2. Placebo-adjusted changes from baseline were statistically significant for each of the 2 mg, 3 mg, and 4 mg guanfacine extended-release tablets randomized treatment groups in both studies, as well as the 1 mg guanfacine extended-release tablets treatment group that was included only in Study 2 (see Table 16).
Dose-responsive efficacy was evident, particularly when data were examined on a weight-adjusted (mg/kg) basis. When evaluated over the dose range of 0.01-0.17 mg/kg/day, clinically relevant improvements were observed beginning at doses in the range 0.05-0.08 mg/kg/day. Doses up to 0.12 mg/kg/day were shown to provide additional benefit.
In the monotherapy trials (Studies 1 and 2), subgroup analyses were performed to identify any differences in response based on gender or age (6-12 vs. 13-17). Analyses of the primary outcome did not suggest any differential responsiveness on the basis of gender. Analyses by age revealed a statistically significant treatment effect only in the 6-12 age subgroup. Due to the relatively small proportion of adolescent patients (ages 13-17) enrolled into these studies (approximately 25%), these data may not have been sufficient to demonstrate efficacy in the adolescent patients. In these studies, patients were randomized to a fixed dose of guanfacine extended-release tablets rather than optimized by body weight. Therefore, some adolescent patients were randomized to a dose that might have resulted in relatively lower plasma guanfacine concentrations compared to the younger patients. Over half (55%) of the adolescent patients received doses of 0.01-0.04 mg/kg. In studies in which systematic pharmacokinetic data were obtained, there was a strong inverse correlation between body weight and plasma guanfacine concentrations.
Study Number | Treatment Group | Primary Efficacy Measure: ADHD-RS-IV Total Score | ||
(Age Range) | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Differencea(95% CI) | |
| Study 1 (6 -17 years) | Guanfacine extended-release tablets 2 mg* Guanfacine extended-release tablets 3 mg* Guanfacine extended-release tablets 4 mg* Placebo | 36.1 (9.99) 36.8 (8.72) 38.4 (9.21) 38.1 (9.34) | -15.9 (1.37) -16.0 (1.38) -18.5 (1.39) -8.5 (1.42) | -7.4 (-11.3, -3.5) -7.5 (-11.4, -3.6) -10.0 (-13.9, -6.1) - |
| Study 2 (6 - 17 years) | Guanfacine extended-release tablets 1 mg*^ Guanfacine extended-release tablets 2 mg* Guanfacine extended-release tablets 3 mg* Guanfacine extended-release tablets 4 mg* Placebo | 41.7 (7.81) 39.9 (8.74) 39.1 (9.22) 40.6 (8.57) 39.3 (8.85) | -19.4 (1.69) -18.1 (1.60) -20.0 (1.64) -20.6 (1.60) -12.7 (1.60) | -6.8 (-11.3, -2.2) -5.4 (-9.9, -0.9) -7.3 (-11.8, -2.8) -7.9 (-12.3, -3.4) - |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
a Difference (drug minus placebo) in least-squares mean change from baseline.
* Doses statistically significantly superior to placebo.
^ The lowest dose of 1 mg used in Study 2 was not randomized to patients weighing more than 50 kg.
Study 3 (313 study) was a double-blind, randomized, placebo-controlled, dose-optimization study, in which efficacy of once daily optimized dosing (morning or evening) with guanfacine extended-release tablets (1 mg, 2 mg, 3 mg and 4 mg), when co-administered with psychostimulants, was evaluated for 8 weeks, in children and adolescents aged 6-17 years with a diagnosis of ADHD, with a sub-optimal response to stimulants (n=455). Patients were started at the 1 mg guanfacine extended-release tablets dose level and were titrated weekly over a 5-week dose-optimization period to an optimal guanfacine extended-release tablets dose not to exceed 4 mg/day based on tolerability and clinical response. The dose was then maintained for a 3-week dose maintenance period before entry to 1 week of dose tapering. Patients took guanfacine extended-release tablets either in the morning or the evening while maintaining their current dose of psychostimulant treatment given each morning. Allowable psychostimulants in the study were ADDERALL XR®, VYVANSE®, CONCERTA®, FOCALIN XR®, RITALIN LA®, METADATE CD®or FDA-approved generic equivalents.
Symptoms of ADHD were evaluated on a weekly basis by clinicians using the ADHD Rating Scale (ADHD-RS-IV), which includes both hyperactive/impulsive and inattentive subscales. The primary efficacy outcome was the change from baseline to endpoint in ADHD-RS-IV total scores. Endpoint was defined as the last post-randomization treatment week prior to dose tapering for which a valid score was obtained (up to Week 8).
Mean reductions in ADHD-RS-IV total scores at endpoint were statistically significantly greater for guanfacine extended-release tablets given in combination with a psychostimulant compared to placebo given with a psychostimulant for Study 3, for both morning and evening guanfacine extended-release tablets dosing (see Table 17). Nearly two-thirds (64.2%) of patients reached optimal doses in the 0.05-0.12 mg/kg/day range.
Study 4 (314 study) was a double-blind, randomized, placebo-controlled, dose-optimization study, in which efficacy of once daily dosing (morning or evening) with guanfacine extended-release tablets (1 mg, 2 mg, 3 mg, and 4 mg) was evaluated for 8 weeks in children aged 6-12 years (n=340).
Signs and symptoms of ADHD were evaluated on a once weekly basis using the clinician administered and scored ADHD Rating Scale (ADHD-RS-IV), which includes both hyperactive/impulsive and inattentive subscales. The primary efficacy outcome was the change from baseline score at endpoint on the ADHD-RS-IV total scores. Endpoint was defined as the last post-randomization treatment week for which a valid score was obtained prior to dose tapering (up to Week 8).
Mean reductions in ADHD-RS-IV total scores at endpoint were statistically significantly greater for guanfacine extended-release tablets compared to placebo in both AM and PM dosing groups of guanfacine extended-release tablets (see Table 17).
Study 5 (312 study) was a 15-week, double-blind, randomized, placebo-controlled, dose-optimization study conducted in adolescents aged 13-17 years (n=314) to evaluate the efficacy and safety of guanfacine extended-release tablets (1-7 mg/day; optimized dose range of 0.05-0.12 mg/kg/day) in the treatment of ADHD as measured by the ADHD Rating Scale-IV (ADHD-RS-IV). Patients receiving guanfacine extended-release tablets showed statistically significantly greater improvement on the ADHD-RS-IV total score compared with patients receiving placebo (see Table 17).
Study 6 (316 study) was a 12-week (for children aged 6-12) or 15-week (for adolescents aged 13-17), randomized, double-blind, parallel-group, placebo- and active-reference, dose-optimization study conducted in pediatric patients (children and adolescents aged 6-17 years old inclusive) (n=337) to assess the efficacy and safety of once-daily dosing (children: 1-4 mg/day, adolescents: 1-7 mg/day; optimized dose range of 0.05 to 0.12 mg/kg/day) in the treatment of ADHD. Guanfacine extended-release tablets were statistically superior to placebo on symptoms of ADHD in patients 6-17 years as measured by change from baseline in ADHD-RS-IV total scores (see Table 17).
Primary Efficacy Measure: ADHD-RS-IV Total Score | ||||
Study Number (Age Range) | Treatment Group | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo- subtracted Differenceb (95% CI) |
| Study 3a (6-17 years) | Guanfacine extended-release tablets 1-4 mg AM* Guanfacine extended-release tablets 1-4 mg PM* Placebo | 37.6 (8.13) 37.0 (7.65) 37.7 (7.75) | -20.3 (0.97) -21.2 (0.97) -15.9 (0.96) | -4.5 (-7.5, -1.4) -5.3 (-8.3, -2.3) - |
| Study 4 (6-12 years) | Guanfacine extended-release tablets 1-4 mg AM* Guanfacine extended-release tablets 1-4 mg PM* Placebo | 41.7 (6.39) 41.6 (6.66) 42.9 (6.29) | -20.0 (1.23) -20.4 (1.19) -10.6 (1.20) | -9.4 (-12.8, -6.0) -9.8 (-13.1, -6.4) - |
| Study 5 (13-17 years) | Guanfacine extended-release tablets 1-7 mg* Placebo | 39.9 (5.57) 40.0 (6.11) | -24.6 (1.06) -18.5 (1.08) | -6.03 (-8.87, -3.19) - |
| Study 6 (6-17 years) | Guanfacine extended-release tablets 1-7 mg* Placebo | 43.1 (5.47) 43.2 (5.60) | -23.89 (1.15) -15.01 (1.16) | -8.88 (-11.94, 5.81) - |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
a Treatment was given in combination with a psychostimulant.
b Difference (drug minus placebo) in least-squares mean change from baseline.
* Doses statistically significantly superior to placebo.
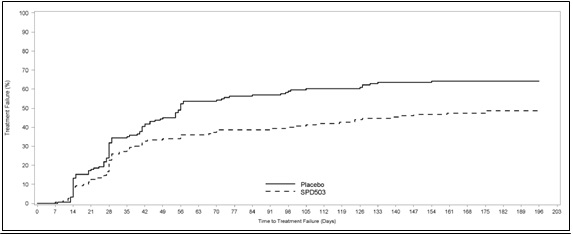
Study 7 (315 study) was a double-blind, placebo-controlled, randomized withdrawal trial in pediatric patients aged 6 to 17 years with DSM-IV-TR diagnosis of ADHD. The study consisted of an open-label phase, including a 7-week dose optimization period to titrate patients to an optimal dose (maximum 4 mg/day for children and 7 mg/day for adolescents; optimized dose range: 0.05 to 0.12 mg/kg/day) and a 6-week dose maintenance period. There were 526 patients included in the open-label phase. Among those, 315 patients who met response criteria in the open-label phase were then randomized (1:1, guanfacine extended-release tablets: placebo) in a 26-week, double-blind, randomized withdrawal phase. The response criteria were defined by ≥30% reduction in ADHD-RS-IV total score and a Clinical Global Impression-Improvement (CGI-I) score of 1 or 2 during the open-label phase. A statistically significantly lower proportion of treatment failures occurred among guanfacine extended-release tablets patients compared to placebo at the end of the randomized withdrawal period . Treatment failure was defined as a ≥50% increase (worsening) in ADHD-RS-IV total score and a ≥2-point increase in Clinical Global Impression-Severity (CGI-S) score. Patients who met the treatment failure criteria on two consecutive visits or discontinued for any reason were classified as treatment failure.

- Recommended dose: 1 mg to 7 mg (0.05-0.12 mg/kg target weight based dose range) once daily in the morning or evening based on clinical response and tolerability ().2.2 Dose Selection
Take guanfacine extended-release tablets orally once daily, either in the morning or evening, at approximately the same time each day. Begin at a dose of 1 mg/day, and adjust in increments of no more than 1 mg/week.
In monotherapy clinical trials, there was dose- and exposure-related clinical improvement as well as risks for several clinically significant adverse reactions (hypotension, bradycardia, sedative events). To balance the exposure-related potential benefits and risks, the recommended target dose range depending on clinical response and tolerability for guanfacine extended-release tablets is 0.05-0.12 mg/kg/day (total daily dose between 1-7 mg) (See Table 1).
Table 1: Recommended Target Dose Range for Therapy with Guanfacine Extended-Release TabletsWeightTarget dose range (0.05 - 0.12 mg/kg/day)25-33.9 kg 2-3 mg/day 34-41.4 kg 2-4 mg/day 41.5-49.4 kg 3-5 mg/day 49.5-58.4 kg 3-6 mg/day 58.5-91 kg 4-7 mg/day >91 kg 5-7 mg/day Doses above 4 mg/day have not been evaluated in children (ages 6-12 years) and doses above 7 mg/day have not been evaluated in adolescents (ages 13-17 years) In the adjunctive trial which evaluated guanfacine extended-release tablets treatment with psychostimulants, the majority of patients reached optimal doses in the 0.05-0.12 mg/kg/day range. Doses above 4 mg/day have not been studied in adjunctive trials.
- Begin at a dose of 1 mg once daily and adjust in increments of no more than 1 mg/week ().2.2 Dose Selection
Take guanfacine extended-release tablets orally once daily, either in the morning or evening, at approximately the same time each day. Begin at a dose of 1 mg/day, and adjust in increments of no more than 1 mg/week.
In monotherapy clinical trials, there was dose- and exposure-related clinical improvement as well as risks for several clinically significant adverse reactions (hypotension, bradycardia, sedative events). To balance the exposure-related potential benefits and risks, the recommended target dose range depending on clinical response and tolerability for guanfacine extended-release tablets is 0.05-0.12 mg/kg/day (total daily dose between 1-7 mg) (See Table 1).
Table 1: Recommended Target Dose Range for Therapy with Guanfacine Extended-Release TabletsWeightTarget dose range (0.05 - 0.12 mg/kg/day)25-33.9 kg 2-3 mg/day 34-41.4 kg 2-4 mg/day 41.5-49.4 kg 3-5 mg/day 49.5-58.4 kg 3-6 mg/day 58.5-91 kg 4-7 mg/day >91 kg 5-7 mg/day Doses above 4 mg/day have not been evaluated in children (ages 6-12 years) and doses above 7 mg/day have not been evaluated in adolescents (ages 13-17 years) In the adjunctive trial which evaluated guanfacine extended-release tablets treatment with psychostimulants, the majority of patients reached optimal doses in the 0.05-0.12 mg/kg/day range. Doses above 4 mg/day have not been studied in adjunctive trials.
- Do not crush, chew or break tablets before swallowing (2.1 General Instruction for UseSwallow tablets whole. Do not crush, chew, or break tablets because this will increase the rate of guanfacine release. Do not administer with high fat meals, due to increased exposure.
- Do not administer with high-fat meals, because of increased exposure ().2.1 General Instruction for UseSwallow tablets whole. Do not crush, chew, or break tablets because this will increase the rate of guanfacine release. Do not administer with high fat meals, due to increased exposure.
- Do not substitute for immediate-release guanfacine tablets on a mg-per-mg basis, because of differing pharmacokinetic profiles ().2.3 Switching from Immediate-Release Guanfacine to Guanfacine Extended-Release Tablets
If switching from immediate-release guanfacine, discontinue that treatment, and titrate with guanfacine extended-release tablets following above recommended schedule.
Do not substitute for immediate-release guanfacine tablets on a milligram-per-milligram basis, because of differing pharmacokinetic profiles. Guanfacine extended-release tablets have significantly reduced Cmax(60% lower), bioavailability (43% lower), and a delayed Tmax(3 hours later) compared to those of the same dose of immediate-release guanfacine [
see Clinical Pharmacology ]. - If switching from immediate-release guanfacine, discontinue that treatment and titrate with guanfacine extended-release tablets as directed ().2.3 Switching from Immediate-Release Guanfacine to Guanfacine Extended-Release Tablets
If switching from immediate-release guanfacine, discontinue that treatment, and titrate with guanfacine extended-release tablets following above recommended schedule.
Do not substitute for immediate-release guanfacine tablets on a milligram-per-milligram basis, because of differing pharmacokinetic profiles. Guanfacine extended-release tablets have significantly reduced Cmax(60% lower), bioavailability (43% lower), and a delayed Tmax(3 hours later) compared to those of the same dose of immediate-release guanfacine [
see Clinical Pharmacology ]. - When discontinuing, taper the dose in decrements of no more than 1 mg every 3 to 7 days to avoid rebound hypertension ().2.5 Discontinuation of Treatment
Following discontinuation of guanfacine extended-release tablets, patients may experience increases in blood pressure and heart rate [
see Warnings and Precautions and Adverse Reactions]. Patients/caregivers should be instructed not to discontinue guanfacine extended-release tablets without consulting their health care provider. Monitor blood pressure and pulse when reducing the dose or discontinuing the drug. Taper the daily dose in decrements of no more than 1 mg every 3 to 7 days to minimize the risk of rebound hypertension.
1 mg, 2 mg, 3 mg and 4 mg extended-release tablets.
Available data with guanfacine over decades of use in pregnant women have not identified a drug- associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. However, use of guanfacine in pregnant women over this time has been infrequent. In animal reproduction studies, rabbits and rats exposed to 3 and 4 times the maximum recommended human dose (MRHD), respectively, showed no adverse outcomes. However, higher doses were associated with reduced fetal survival and maternal toxicity (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Reproduction studies conducted in rats have shown that guanfacine crosses the placenta. However, administration of guanfacine to rabbits and rats during organogenesis at 3 (rabbit) and 4 (rat) times the MRHD of 0.12 mg/kg/day on a mg/m2 basis resulted in no evidence of harm to the fetus. Higher doses (13.5 times the MRHD in both rabbits and rats) were associated with reduced fetal survival and maternal toxicity.
Guanfacine extended-release tablets are contraindicated in patients with a history of a hypersensitivity reaction to guanfacine extended-release tablets or its inactive ingredients, or other products containing guanfacine. Rash and pruritus have been reported.
- Hypotension, bradycardia, syncope: Titrate slowly and monitor vital signs frequently in patients at risk for hypotension, heart block, bradycardia, syncope, cardiovascular disease, vascular disease, cerebrovascular disease or chronic renal failure. Measure heart rate and blood pressure prior to initiation of therapy, following dose increases, and periodically while on therapy. Avoid concomitant use of drugs with additive effects unless clinically indicated. Advise patients to avoid becoming dehydrated or overheated ().5.1 Hypotension, Bradycardia, and Syncope
Treatment with guanfacine extended-release tablets can cause dose-dependent decreases in blood pressure and heart rate. Decreases were less pronounced over time of treatment. Orthostatic hypotension and syncope have been reported [
see Adverse Reactions ].Measure heart rate and blood pressure prior to initiation of therapy, following dose increases, and periodically while on therapy. Titrate guanfacine extended-release tablets slowly in patients with a history of hypotension, and those with underlying conditions that may be worsened by hypotension and bradycardia; e.g., heart block, bradycardia, cardiovascular disease, vascular disease, cerebrovascular disease, or chronic renal failure. In patients who have a history of syncope or may have a condition that predisposes them to syncope, such as hypotension, orthostatic hypotension, bradycardia, or dehydration, advise patients to avoid becoming dehydrated or overheated. Monitor blood pressure and heart rate, and adjust dosages accordingly in patients treated concomitantly with antihypertensive or other drugs that can reduce blood pressure or heart rate or increase the risk of syncope.
- Sedation and somnolence: Occur commonly with guanfacine extended-release tablets Consider the potential for additive sedative effects with CNS depressant drugs. Caution patients against operating heavy equipment or driving until they know how they respond to guanfacine extended-release tablets ().5.2 Sedation and Somnolence
Somnolence and sedation were commonly reported adverse reactions in clinical
studies [see Adverse Reactions ].Before using guanfacine extended-release tablets with other centrally active depressants, consider the potential for additive sedative effects. Caution patients against operating heavy equipment or driving until they know how they respond to treatment with guanfacine extended-release tablets. Advise patients to avoid use with alcohol. - Cardiac Conduction Abnormalities: May worsen sinus node dysfunction and atrioventricular (AV) block, especially in patients taking other sympatholytic drugs. Titrate slowly and monitor vital signs frequently ().5.3 Cardiac Conduction Abnormalities
The sympatholytic action of guanfacine extended-release tablets may worsen sinus node dysfunction and atrioventricular (AV) block, especially in patients taking other sympatholytic drugs. Titrate guanfacine extended-release tablets slowly and monitor vital signs frequently in patients with cardiac conduction abnormalities or patients concomitantly treated with other sympatholytic drugs.
- Rebound Hypertension: Abrupt discontinuation of guanfacine extended-release tablets can lead to clinically significant and persistent rebound hypertension. Subsequent hypertensive encephalopathy was also reported. To minimize the risk of rebound hypertension upon discontinuation, the total daily dose of guanfacine extended-release tablets should be tapered in decrements of no more than 1 mg every 3 to 7 days ().5.4 Rebound Hypertension
In post marketing experience, abrupt discontinuation of guanfacine extended-release tablets has resulted in clinically significant and persistent rebound hypertension above baseline levels and increases in heart rate. Hypertensive encephalopathy has also been reported in association with rebound hypertension with both guanfacine extended-release tablets and immediate release guanfacine
[see Adverse Reactions ].In these cases, high-dosage guanfacine was discontinued; concomitant stimulant use was also reported, which may potentially increase hypertensive response upon abrupt discontinuation of guanfacine. Children commonly have gastrointestinal illnesses that lead to vomiting, and a resulting inability to take medications, so they may be especially at risk for rebound hypertension.To minimize the risk of rebound hypertension upon discontinuation, the total daily dose of guanfacine extended-release tablets should be tapered in decrements of no more than 1 mg every 3 to 7 days
[see Dosage and Administration ].Blood pressure and heart rate should be monitored when reducing the dose or discontinuing guanfacine extended-release tablets. If abrupt discontinuation occurs (especially with concomitant stimulant use), patients should be closely followed for rebound hypertension.