Hailey 24 Fe Prescribing Information
Hailey 24 Fe is contraindicated in females who are known to have or develop the following conditions:
• A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:• Smoke, if over age 35[see Boxed Warning and Warnings and Precautions ]• Have deep vein thrombosis or pulmonary embolism, now or in the past[see Warnings and Precautions ]• Have inherited or acquired hypercoagulopathies[see Warnings and Precautions ]• Have cerebrovascular disease[see Warnings and Precautions ]• Have coronary artery disease[see Warnings and Precautions ]• Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation)[see Warnings and Precautions ]• Have uncontrolled hypertension[see Warnings and Precautions ]• Have diabetes mellitus with vascular disease[see Warnings and Precautions ]• Have headaches with focal neurological symptoms or have migraine headaches with aura[see Warnings and Precautions ]o Women over age 35 with any migraine headaches[see Warnings and Precautions ]
• Liver tumors, benign or malignant, or liver disease[see Warnings and Precautions ]• Undiagnosed abnormal uterine bleeding [see Warnings and Precautions ]● Current diagnosis of, or history of, breast cancer, which may be hormone sensitive [see Warnings and Precautions ]● Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations [see Warnings and Precautions ]
A high risk of arterial or venous thrombotic diseases
• Liver tumors or liver disease• Undiagnosed abnormal uterine bleeding• Breast cancer• Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir
Warnings and Precautions, Malignant Neoplasms (
Some studies suggest that COCs are associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there is controversy about the extent to which these findings may be due to differences in sexual behavior and other factors.
Hailey 24 Fe is indicated for use by women to prevent pregnancy
In an active-controlled clinical trial, 743 women 18 to 45 years of age were studied to assess the efficacy of norethindrone acetate and ethinyl estradiol tablets and ferrous fumarate tablets, for up to six 28-day cycles. The racial demographic of women randomized to norethindrone acetate and ethinyl estradiol tablets and ferrous fumarate tablets was: 69.5% Caucasian, 15.5% African-American, 10.4% Hispanic, 2.3% Asian and 2.3% Native American/Other. Women with body mass index (BMI) greater than 35 mg/m2were excluded from the study. The weight range for those women treated was 90 to 260 pounds, with a mean weight of 147 pounds. Among the women in the study randomized to norethindrone acetate and ethinyl estradiol tablets and ferrous fumarate tablets, 38.9% had not used hormonal contraception immediately prior to enrolling in this study.
A total of 583 women completed 6 cycles of treatment. There were a total of 5 on-treatment pregnancies among women aged 18 to 45 years in 3,565 treatment cycles during which no back-up contraception was used. The Pearl Index for norethindrone acetate and ethinyl estradiol tablets and ferrous fumarate tablets was 1.82 (95% confidence interval 0.59 to 4.25).
The efficacy of Hailey 24 Fe in women with a body mass index (BMI) of > 35 kg/m2 has not been evaluated.
• Take one tablet by mouth at the same time every day for 28 days ()2.1 How to Start Hailey 24 FeHailey 24 Fe is dispensed in a blister
[see How Supplied/Storage and Handling ].Hailey 24 Fe may be started using either a Day 1 start or a Sunday start (see Table 1). For the first cycle of a Sunday Start regimen, an additional method of contraception must be used until after the first 7 consecutive days of administration.• Take tablets in the order directed on the blister pack ()2.1 How to Start Hailey 24 FeHailey 24 Fe is dispensed in a blister
[see How Supplied/Storage and Handling ].Hailey 24 Fe may be started using either a Day 1 start or a Sunday start (see Table 1). For the first cycle of a Sunday Start regimen, an additional method of contraception must be used until after the first 7 consecutive days of administration.• Hailey 24 Fe may be administered without regard to meals ()12.3 PharmacokineticsAbsorptionNorethindrone acetate appears to be completely and rapidly deacetylated to norethindrone after oral administration, because the disposition of norethindrone acetate is indistinguishable from that of orally administered norethindrone. Norethindrone acetate and ethinyl estradiol are rapidly absorbed from norethindrone acetate and ethinyl estradiol tablets and ferrous fumarate tablets, with maximum plasma concentrations of norethindrone and ethinyl estradiol occurring 1 to 4 hours postdose. Both are subject to first-pass metabolism after oral dosing, resulting in an absolute bioavailability of approximately 64% for norethindrone and 43% for ethinyl estradiol.
The plasma norethindrone and ethinyl estradiol pharmacokinetics following single- and multiple-dose administrations of norethindrone acetate and ethinyl estradiol tablets and ferrous fumarate tablets in 17 healthy female volunteers are provided in Figures 2 and 3, and Table 3.
Following multiple-dose administration of norethindrone acetate and ethinyl estradiol tablets and ferrous fumarate tablets, mean maximum concentrations of norethindrone and ethinyl estradiol were increased by 95% and 27%, respectively, as compared to single-dose administration. Mean norethindrone and ethinyl estradiol exposures (AUC values) were increased by 164% and 51% respectively, as compared to single-dose administration of norethindrone acetate and ethinyl estradiol tablets and ferrous fumarate tablets.
Steady-state with respect to norethindrone was reached by Day 17 and steady-state with respect to ethinyl estradiol was reached by Day 13.
Mean SHBG concentrations were increased by 150% from baseline (57.5 nmol/L) to 144 nmol/L at steady-state.
Figure 2. Mean Plasma Norethindrone Concentration-Time Profiles Following Single- and Multiple-Dose Oral Administration of Norethindrone Acetate and Ethinyl Estradiol Tablets and Ferrous Fumarate Tablets to Healthy Female Volunteers Under Fasting Condition (n = 17)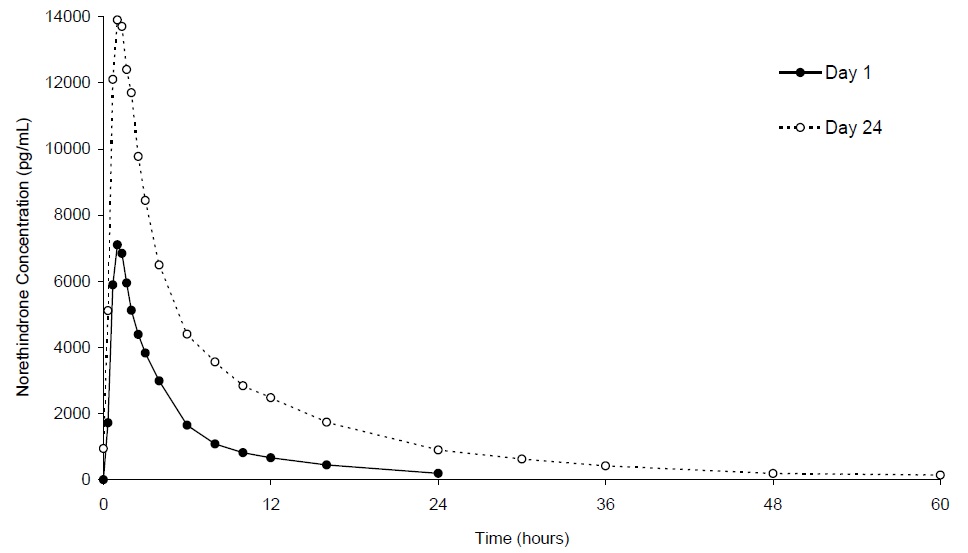 Figure 3. Mean Plasma Ethinyl Estradiol Concentration-Time Profiles Following Single- and Multiple-Dose Oral Administration of Norethindrone Acetate and Ethinyl Estradiol Tablets and Ferrous Fumarate Tablets to Healthy Female Volunteers Under Fasting Condition (n = 17)
Figure 3. Mean Plasma Ethinyl Estradiol Concentration-Time Profiles Following Single- and Multiple-Dose Oral Administration of Norethindrone Acetate and Ethinyl Estradiol Tablets and Ferrous Fumarate Tablets to Healthy Female Volunteers Under Fasting Condition (n = 17)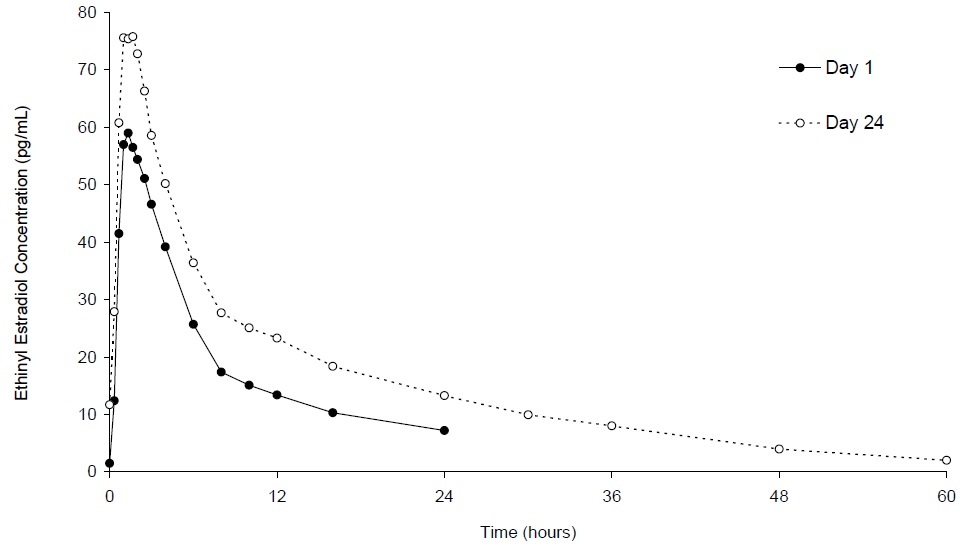 Table 3. Summary of Norethindrone (NE) and Ethinyl Estradiol (EE) Pharmacokinetics Following Single- and Multiple-Dose Oral Administration of Norethindrone Acetate and Ethinyl Estradiol Tablets and Ferrous Fumarate Tablets to Healthy Female Volunteers Under Fasting Condition (n = 17)
Table 3. Summary of Norethindrone (NE) and Ethinyl Estradiol (EE) Pharmacokinetics Following Single- and Multiple-Dose Oral Administration of Norethindrone Acetate and Ethinyl Estradiol Tablets and Ferrous Fumarate Tablets to Healthy Female Volunteers Under Fasting Condition (n = 17)Regimen
Analyte
Arithmetic Meana(% CV) by Pharmacokinetic Parameter
Cmax
(pg/mL)
tmax
(hr)
AUC(0 to 24)
(pg/mL•h)
Cmin
(pg/mL)
t½
(hr)
Cavg
(pg/mL)
Day 1
(Single Dose)
NE
8420
(31)
1.0
(0.7 to 4.0)
33390
(40)
--
--
--
EE
64.5
(27)
1.3
(0.7 to 4.0)
465.4
(26)
--
--
--
SHBG
--
--
--
57.5
(37)b
--
--
Day 24 (Multiple Dose)
NE
16400
(26)
1.3
(0.7 to 4.0)
88160
(30)
880
(51)
8.4
3670
(30)
EE
81.9
(24)
1.7
(1.0 to 2.0)
701.3
(28)
11.4
(43)
14.5
29.2
(28)
SHBG
--
--
--
144
(24)
--
--
Cmax= Maximum plasma concentration
tmax= Time of Cmax
Cmin= minimum plasma concentration at steady-state
AUC(0 to 24)= Area under plasma concentration versus time curve from 0 to 24 hours
t½= Apparent first-order terminal elimination half-life
Cavg= Average plasma concentration = AUC(0 to 24)/24
% CV = Coefficient of Variation (%)
SHBG = Sex Hormone Binding Globulin (nmol/L)
aThe harmonic mean (0.693/mean apparent elimination rate constant) is reported for t½,and the median (range) is reported for tmax.
bThe SHBG concentration reported here is the pre-dose concentration.
Food EffectA single-dose administration of norethindrone acetate and ethinyl estradiol tablets and ferrous fumarate tablet with food decreased the maximum concentration of norethindrone by 11% and increased the extent of absorption by 27% and decreased the maximum concentration of ethinyl estradiol by 30% but not the extent of absorption.
DistributionVolume of distribution of norethindrone and ethinyl estradiol ranges from 2 to 4 L/kg. Plasma protein binding of both steroids is extensive (>95%); norethindrone binds to both albumin and SHBG, whereas ethinyl estradiol binds only to albumin. Although ethinyl estradiol does not bind to SHBG, it induces SHBG synthesis.
MetabolismNorethindrone undergoes extensive biotransformation, primarily via reduction, followed by sulfate and glucuronide conjugation. The majority of metabolites in the circulation are sulfates, with glucuronides accounting for most of the urinary metabolites.
Ethinyl estradiol is also extensively metabolized, both by oxidation and by conjugation with sulfate and glucuronide. Sulfates are the major circulating conjugates of ethinyl estradiol and glucuronides predominate in urine. The primary oxidative metabolite is 2-hydroxy ethinyl estradiol, formed by the CYP3A4 isoform of cytochrome P450. Part of the first-pass metabolism of ethinyl estradiol is believed to occur in gastrointestinal mucosa. Ethinyl estradiol may undergo enterohepatic circulation.
ExcretionNorethindrone and ethinyl estradiol are excreted in both urine and feces, primarily as metabolites. Plasma clearance values for norethindrone and ethinyl estradiol are similar (approximately 0.4 L/hr/kg). Steady-state elimination half-lives of norethindrone and ethinyl estradiol following administration of norethindrone acetate and ethinyl estradiol tablets and ferrous fumarate tablets are approximately 8 hours and 14 hours, respectively.

Figure 1.jpg 
Figure 2.jpg
Hailey® 24 Fe (norethindrone acetate and ethinyl estradiol tablets, USP and ferrous fumarate tablets, USP) is available in blister packs.
Each blister pack (28 tablets) contains in the following order:
• 24 white to off-white, round, flat faced beveled edge, uncoated tablets debossed with ‘16’ on one side and ‘G’ on the other side and each containing 1 mg norethindrone acetate, USP and 20 mcg ethinyl estradiol, USP.• 4 brown to dark brown, round, flat faced beveled edge, uncoated tablets debossed with ‘17’ on one side and ‘G’ on the other side and each containing 75 mg ferrous fumarate, USP. The ferrous fumarate tablets do not serve any therapeutic purpose.
• Lactation: Advise use of another contraceptive method. Hailey 24 Fe can decrease milk production. (8.2)