Heparin Sodium In Sodium Chloride - Heparin Sodium In Sodium Chloride injection
(Heparin Sodium In Sodium Chloride)Heparin Sodium In Sodium Chloride - Heparin Sodium In Sodium Chloride injection Prescribing Information
Heparin Sodium in 0.9% Sodium Chloride Injection at the concentration of 2 USP units/mL is indicated as an anticoagulant to maintain catheter patency.
HEPARIN SODIUM IN 0.9% SODIUM CHLORIDE INJECTION is a sterile, single-dose, clear, nonpyrogenic solution available as:
- Heparin Sodium 1,000 USP units per 500 mL (2 USP units per mL) in 0.9% Sodium Chloride Injection.
- Heparin Sodium 2,000 USP units per 1,000 mL (2 USP units per mL) in 0.9% Sodium Chloride Injection.
The use of HEPARIN SODIUM IN 0.9% SODIUM CHLORIDE INJECTION is contraindicated in patients with the following conditions:
- Uncontrollable active bleeding state except when this is due to disseminated intravascular coagulation [see Warnings and Precautions (5.2)]
- History of heparin-induced thrombocytopenia (HIT) or heparin-induced thrombocytopenia and thrombosis (HITT) [see Warnings and Precautions (5.3)]
- Severe thrombocytopenia [see Warnings and Precautions ]
- Known hypersensitivity to heparin or pork products (e.g., anaphylactoid reactions) [see Warnings and Precautions (5.5) and Adverse Reactions (6.1)]
The following serious adverse reactions are described elsewhere in the labeling:
Hemorrhage
[see Warnings and Precautions (5.2)]
Heparin-Induced Thrombocytopenia and Heparin-Induced Thrombocytopenia and Thrombosis
[see Warnings and Precautions (5.3)]Thrombocytopenia
[see Warnings and Precautions (5.4)]Hypersensitivity Reactions
[see Warnings and Precautions (5.5)]Heparin Resistance
[see Warnings and Precautions (5.6)]Hyperkalemia
[see Warnings and Precautions (5.7)]Elevations of Serum Aminotransferases
[see Warnings and Precautions (5.8)]
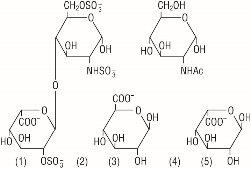
Heparin is a heterogenous group of straight-chain anionic mucopolysaccharides, called glycosaminoglycans having anticoagulant properties. It is composed of polymers of alternating derivations of alpha-L-iduronic acid 2-sulfate (1), 2-deoxy-2-sulfamino-alpha-D-glucose 6-sulfate (2), beta-D-glucuronic acid (3), 2-acetamido-2-deoxy-alpha-D-glucose (4), and alpha-L-iduronic acid (5).
Structure of Heparin Sodium (representative subunits):
Heparin Sodium in 0.9% Sodium Chloride Injection is a sterile, single-dose, clear, nonpyrogenic solution prepared from Heparin Sodium USP (derived from porcine intestinal mucosa and standardized for use as an anticoagulant) in 0.9% Sodium Chloride Injection. It is to be administered by intravenous injection. The potency is determined by a biological assay using a USP reference standard based on units of heparin activity per milligram.
Each 100 mL contains 200 USP Units Heparin Sodium, 0.43 g Dibasic Sodium Phosphate•7H2O USP and 0.037 g Citric Acid Anhydrous USP as a buffer system, 0.9 g Sodium Chloride USP, and Water for Injection USP qs.
pH: 7.0 (6.8-7.2); Calculated Osmolarity: 360 mOsmol/liter
Concentration of Electrolytes (mEq/liter): Sodium 186; Chloride 154;
Phosphate (HPO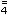 ) 32; Citrate 6
) 32; Citrate 6
The plastic container is made from a multilayered film specifically developed for parenteral drugs. It contains no plasticizers and exhibits virtually no leachables. The solution contact layer is a rubberized copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during administration. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
The plastic container is not made with natural rubber latex, PVC or DEHP.
The closure system has two ports; the one for the administration set has a tamper evident plastic protector.
Heparin interacts with the naturally occurring plasma protein, Antithrombin III, to induce a conformational change, which markedly enhances the serine protease activity of Antithrombin III, thereby inhibiting the activated coagulation factors involved in the closing sequence, particularly Xa and IIa. Small amounts of heparin inhibit Factor Xa, and larger amounts inhibit thrombin (Factor IIa). Heparin also prevents the formation of a stable fibrin clot by inhibiting the activation of the fibrin stabilizing factor. Heparin does not have fibrinolytic activity; therefore, it will not lyse existing clots.