Hydrochlorothiazide
Hydrochlorothiazide Prescribing Information
Hydrochlorothiazide capsules, USP are indicated in the management of hypertension either as the sole therapeutic agent, or in combination with other antihypertensives. Unlike potassium sparing combination diuretic products, hydrochlorothiazide capsules, USP may be used in those patients in whom the development of hyperkalemia cannot be risked, including patients taking ACE inhibitors.
Edema during pregnancy may arise from pathological causes or from the physiologic and mechanical consequences of pregnancy. Diuretics are indicated in pregnancy when edema is due to pathologic causes, just as they are in the absence of pregnancy. Dependent edema in pregnancy resulting from restriction of venous return by the expanded uterus is properly treated through elevation of the lower extremities and use of support hose; use of diuretics to lower intravascular volume in this case is illogical and unnecessary. There is hypervolemia during normal pregnancy which is harmful to neither the fetus nor the mother (in the absence of cardiovascular disease), but which is associated with edema, including generalized edema in the majority of pregnant women. If this edema produces discomfort, increased recumbency will often provide relief. In rare instances this edema may cause extreme discomfort which is not relieved by rest. In these cases a short course of diuretics may provide relief and may be appropriate.
Hydrochlorothiazide is contraindicated in patients with anuria. Hypersensitivity to this product or other sulfonamide derived drugs is also contraindicated.
The adverse reactions associated with hydrochlorothiazide have been shown to be dose related. In controlled clinical trials, the adverse events reported with doses of 12.5 mg hydrochlorothiazide once daily were comparable to placebo. The following adverse reactions have been reported for doses of hydrochlorothiazide 25 mg and greater and, within each category, are listed in the order of decreasing severity.
Warning signs or symptoms of fluid and electrolyte imbalance include dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
Hypokalemia may develop, especially with brisk diuresis when severe cirrhosis is present, during concomitant use of corticosteroid or adrenocorticotropic hormone (ACTH) or after prolonged therapy. Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Hypokalemia and hypomagnesemia can provoke ventricular arrhythmias or sensitize or exaggerate the response of the heart to the toxic effects of digitalis. Hypokalemia may be avoided or treated by potassium supplementation or increased intake of potassium rich foods.
Dilutional hyponatremia is life-threatening and may occur in edematous patients in hot weather; appropriate therapy is water restriction rather than salt administration, except in rare instances when the hyponatremia is life-threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
When given concurrently the following drugs may interact with thiazide diuretics:
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg, respectively, prior to conception and throughout gestation.
Safety and effectiveness in pediatric patients have not been established.
A greater blood pressure reduction and an increase in side effects may be observed in the elderly (i.e., > 65 years) with hydrochlorothiazide. Starting treatment with the lowest available dose of hydrochlorothiazide (12.5 mg) is therefore recommended. If further titration is required, 12.5 mg increments should be utilized.
Whenever adverse reactions are moderate or severe, thiazide dosage should be reduced or therapy withdrawn.
Warning signs or symptoms of fluid and electrolyte imbalance include dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
Hypokalemia may develop, especially with brisk diuresis when severe cirrhosis is present, during concomitant use of corticosteroid or adrenocorticotropic hormone (ACTH) or after prolonged therapy. Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Hypokalemia and hypomagnesemia can provoke ventricular arrhythmias or sensitize or exaggerate the response of the heart to the toxic effects of digitalis. Hypokalemia may be avoided or treated by potassium supplementation or increased intake of potassium rich foods.
Dilutional hyponatremia is life-threatening and may occur in edematous patients in hot weather; appropriate therapy is water restriction rather than salt administration, except in rare instances when the hyponatremia is life-threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
When given concurrently the following drugs may interact with thiazide diuretics:
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg, respectively, prior to conception and throughout gestation.
Safety and effectiveness in pediatric patients have not been established.
A greater blood pressure reduction and an increase in side effects may be observed in the elderly (i.e., > 65 years) with hydrochlorothiazide. Starting treatment with the lowest available dose of hydrochlorothiazide (12.5 mg) is therefore recommended. If further titration is required, 12.5 mg increments should be utilized.
Hydrochlorothiazide (hydrochlorothiazide, USP 12.5 mg) is the 3,4-dihydro derivative of chlorothiazide. Its chemical name is 6-chloro-3,4-dihydro-2
7H
8ClN
3O
4S
2; its molecular weight is 297.74; and its structural formula is:
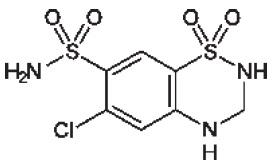
It is a white, or practically white, crystalline powder which is slightly soluble in water, but freely soluble in sodium hydroxide solution.
Hydrochlorothiazide capsules, USP are supplied as 12.5 mg capsules for oral use.