Hydrochlorothiazide
Hydrochlorothiazide Prescribing Information
Hydrochlorothiazide tablets are indicated as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy.
Hydrochlorothiazide tablets have also been found useful in edema due to various forms of renal dysfunction such as nephrotic syndrome, acute glomerulonephritis, and chronic renal failure.
Hydrochlorothiazide tablets are indicated in the management of hypertension either as the sole therapeutic agent or to enhance the effectiveness of other antihypertensive drugs in the more severe forms of hypertension.
Routine use of diuretics during normal pregnancy is inappropriate and exposes mother and fetus to unnecessary hazard. Diuretics do not prevent development of toxemia of pregnancy, and there is no satisfactory evidence that they are useful in the treatment of toxemia.
Edema during pregnancy may arise from pathologic causes or from the physiologic and mechanical consequences of pregnancy. Thiazides are indicated in pregnancy when edema is due to pathologic causes, just as they are in the absence of pregnancy (see
Studies in which hydrochlorothiazide was orally administered to pregnant mice and rats during their respective periods of major organogenesis at doses up to 3,000 and 1,000 mg hydrochlorothiazide/kg, respectively provided no evidence of harm to the fetus.
There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Thiazides cross the placental barrier and appear in cord blood. There is a risk of fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in adults.
Therapy should be individualized according to patient response. Use the smallest dosage necessary to achieve the required response.
Anuria.
Hypersensitivity to this product or to other sulfonamide-derived drugs.
The following adverse reactions have been reported and, within each category, are listed in order of decreasing severity.
Use with caution in severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with imparied renal function.
Thiazides should be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
Thiazides may add to or potentiate the action of other antihypertensive drugs.
Sensitivity reactions may occur in patients with or without a history of allergy or bronchial asthma.
The possibility of exacerbation or activation of systemic lupus erythematosus has been reported.
Lithium generally should not be given with diuretics (see PRECAUTIONS, Drug Interactions).
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
Use with caution in severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with imparied renal function.
Thiazides should be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
Thiazides may add to or potentiate the action of other antihypertensive drugs.
Sensitivity reactions may occur in patients with or without a history of allergy or bronchial asthma.
The possibility of exacerbation or activation of systemic lupus erythematosus has been reported.
Lithium generally should not be given with diuretics (see PRECAUTIONS, Drug Interactions).
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle-closure glaucoma may include a history of sulfonamide or penicillin allergy.
Whenever adverse reactions are moderate or severe, thiazide dosage should be reduced or therapy withdrawn.
Hydrochlorothiazide is associated with an increased risk of non-melanoma skin cancer. In a study conducted in the Sentinel System, increased risk was predominantly for squamous cell carcinoma (SCC) and in white patients taking large cumulative doses. The increased risk for SCC in the overall population was approximately 1 additional case per 16,000 patients per year, and for white patients taking a cumulative dose of ≥50,000 mg the risk increase was approximately 1 additional SCC case for every 6,700 patients per year.
When given concurrently the following drugs may interact with thiazide diuretics.
Hydrochlorothiazide is a diuretic and antihypertensive. It is the 3,4-dihydro derivative of chlorothiazide. Its chemical name is 6-chloro-3,4-dihydro-2
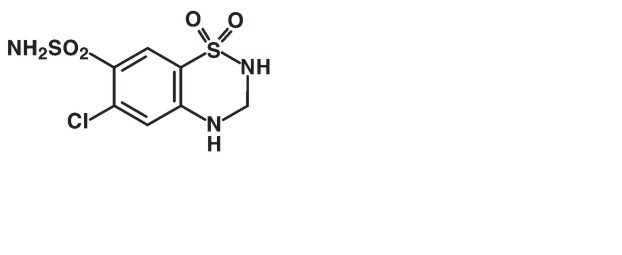
It is a white, or practically white, crystalline powder with a molecular weight of 297.74 which is slightly soluble in water, freely soluble in sodium hydroxide solution, in n-butylamine and in dimethylformamide; sparingly soluble in methanol; insoluble in ether, in chloroform and in dilute mineral acids.
Each tablet for oral administration contains 12.5 mg, 25 mg or 50 mg of hydrochlorothiazide respectively. In addition, each tablet contains following inactive ingredients: anhydrous lactose, D&C Yellow No.10 aluminum lake, FD&C Red No. 40 aluminum lake, magnesium stearate, pregelatinized starch and sodium starch glycolate.