Imuflex Wb-rp Blood Bag System With Integral Whole Blood Leukocyte Reduction Filter (removing Platelets) With Diversion Blood Sampling Arm Anticoagulant Citrate Phosphate Dextrose (cpd) And Optisol (as-5) Red Cell Preservative
(Anticoagulant Citrate Phosphate Dextrose (Cpd) And As-5 Red Cell Preservative)Imuflex Wb-Rp Blood Bag System With Integral Whole Blood Leukocyte Reduction Filter (Removing Platelets) With Diversion Blood Sampling Arm Anticoagulant Citrate Phosphate Dextrose (Cpd) And Optisol (As-5) Red Cell Preservative Prescribing Information
1.1. Read these instructions carefully before use.
1.2. Rx ONLY.
1.3. Intended for the collection, processing and preservation of Whole Blood and blood components. Not intended for direct intravenous infusion.
1.4. For the collection of 500 mL ±10% Whole Blood.
1.5. Integral Diversion Blood Sampling Arm is intended to divert and obtain donor samples for laboratory testing prior to collection of the Whole Blood unit.
1.6. Integral filter unit intended for leukocyte reduction of Whole Blood up to 8 hours after blood collection when Whole Blood is stored at ambient temperature or cooled towards 1-10°C (transport temperature).
1.7. For further processing, use standard component processing techniques.
2.1. To open blister package, peel cover film back 4/5 of its length.
2.2. Prepare the blood bag following your institution's standard operating procedures.
2.2.1. Materials Needed:
- VENOJECT®@Tube Holder (code P-1316R) or equivalent
- VENOJECT@Multi-Sample Luer Adapter (code MN*2000T) or equivalent
- Evacuated blood collection tubes (glass or plastic)
2.3. Make a loose knot in the donor tubing below the "Y" and CLIKTIP® (inline closure device) unless alternate methods are used to seal the tubing at the end of collection.
2.4. Temporarily clamp donor tubing between the phlebotomy needle and the "Y".
2.5. Close the White Clamp below the diversion pouch.
2.6. Assemble the luer adapter and the tube holder.
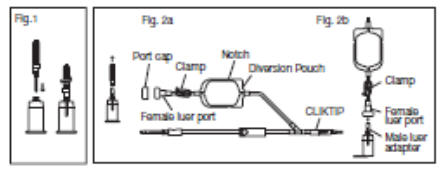
2.6.1. Connect the VENOJECT@Multi-Sample Luer Adapter to the VENOJECT@Tube Holder (or equivalent) (Fig. 1).
2.6.2. Twist and snap to remove the blue port cap at the end of the Diversion Blood Sampling Arm (Fig. 2a).
2.6.3. Insert the Holder/Luer assembly in the female luer port (Fig. 2b).
2.6.4. NOTE: Alternatively, steps 2.6.1., 2.6.2., and 2.6.3. (above) may be performed at any time during bag preparation or after the blood is collected into the diversion pouch.
2.7. Suspend the collection bag as far as possible below the donor's arm.
2.8. Apply blood pressure cuff or tourniquet to donor's arm. Disinfect site of phlebotomy. If blood pressure cuff is used, inflate to approximately 60 mmHg.
2.9. Remove the needle cover and perform phlebotomy. Remove the temporary clamp on the donor tubing to permit blood flow into the Diversion Blood Sampling Arm pouch.
2.9.1.
2.9.2.
2.10. Secure the needle safety device in place following the device instructions provided on the reverse side.
2.11. Secure donor tubing to donor's arm.
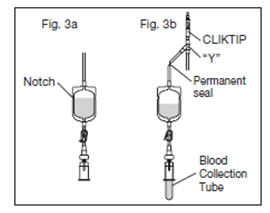
2.12. Position the diversion pouch with the notches up and the Tube Holder/Luer Adapter assembly (or port cap) down. When the level of blood in the pouch is approximately in line with the notches, the diversion pouch is full (Fig. 3a).
2.12.1.
2.13. Permanently seal the tubing between the "Y" and the diversion pouch to maintain a closed system using an aluminum clip or a tube sealer approved for use with tubing connected to a donor (Fig. 3b).
2.13.1.
2.14. To initiate blood flow into the collection bag, break the CLIKTIP between the "Y" and the collection bag.
2.15. To avoid clot formation, collect samples as soon as possible from the diversion pouch as follows (Fig. 3b).
2.15.1.
2.15.2. Open the White Clamp on the tubing below the pouch to open the pathway for sampling.
2.15.3. Position the diversion pouch with the notches up and the Tube Holder/Luer Adapter assembly downward. Assure that any air in the pouch is at the top and will not enter the blood collection tubes.
2.15.4. Insert blood collection tube firmly into the tube holder; when full, remove sample tube from holder. Repeat to collect additional samples.
2.15.5.
A second seal must be made between the diversion pouch and the permanent seal prior to removing the pouch.
2.16. Mix blood with anticoagulant in the collection bag and continue to mix at several intervals during collection and immediately after collection. If using an automated mixer, follow manufacturer's instructions.
2.17. Collect labeled volume of blood 500 mL ±10%.
2.18. When the desired amount of blood has been collected, seal the tubing or tighten the loose knot (white knot) prepared in Step 2.3. Make a second seal between the first seal or knot and the "Y". Various methods may be used to seal tubing.
2.19. Release pressure on the donor's arm and remove the needle into the needle safety device following the device instructions provided on the reverse side. Sever the donor tubing between the two seals previously made below the CLIKTIP and "Y".
2.19.1.
2.20. Seal and remove donor tubing from collection bag or strip donor tubing as follows:
2.20.1. To obtain a quality control prefiltration sample, strip blood from donor tubing into collection bag, mix well, and allow tubing to refill; repeat once. To prevent the blood from clotting in the tubing, work quickly as possible. Leave an adequate length of tubing containing the well-mixed anticoagulated whole blood attached to the collection bag.
2.20.2. Or, to maximize collection recovery, strip the tubing, mix well and seal tubing close to the collection bag without refilling. To prevent the blood from clotting in the tubing, work quickly as possible. Remove tubing from collection bag.
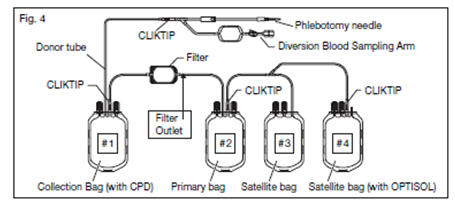
2.21. The blood filtration is executed according to the following operation. (see
2.21.1.
2.21.2.

2.22. Mix the unfiltered whole blood unit by inverting the collection bag #1 (pre-filter bag) several times.
2.23. Hang the collection bag #1 and extend the filtration set to full length. Verify that the filter is vertical and ensure all tubing is freely suspended without kinks.
2.24. Break the CLIKTIP at the outlet of the collection bag #1.
2.24.1. The filter housing will begin to fill with blood and expand on the non-textured side.
2.24.2. Blood will flow across the filter pads and expand the textured side of the filter housing.
2.24.3. Air from the bag and filter will be forced into the primary bag #2 (post-filtration bag).
2.24.3.1.
2.25. Filtration ends when the filter has emptied and the housing has collapsed onto the filter pads.
2.25.1.
2.26. Gently squeeze the primary bag #2 to expel the air in the bag up the numbered segment tubing line and into the filter. To ensure ease of air removal ensure primary bag #2 is below the filter outlet.
2.27. Fill the numbered segment tubing with blood while expelling the air.
2.28. Clamp the blood filled tubing before blood enters the filter.
2.29. Seal the tubing as close as possible to the filter outlet and properly dispose of the filter and collection bag #1.
2.30. Strip the post filter tubing into the primary bag #2, mix well, and allow tubing to refill; repeat once. Make an appropriate number of segments of anticoagulated blood for testing by sealing on or near the X marks. Leave segments attached to the filtered whole blood unit.
2.31. The time between Whole Blood collection and component separation may vary depending on both the blood bag system and processing options selected.
2.31.1.
2.31.2.
2.31.3.
2.32. Follow your institution's standard component processing procedures to prepare components.
2.33. Centrifuge filtered whole blood unit to separate red cells from plasma.
2.34. Break the CLIKTIP of primary bag #2 and transfer leukocytes-reduced plasma into satellite bag #3. Clamp transfer tubing of satellite bag.
2.35. Break the CLIKTIP of OPTISOL Solution bag #4 and drain contents into primary bag #2 containing red blood cells. Seal tubing of primary bag in two places, and cut between seals and separate from satellite bag(s).
2.35.1.
2.36. Invert the red blood cell – OPTISOL mixture several times to assure the final product is well suspended.
2.37. Store AS-5 Red Blood Cells, Leukocytes Reduced between 1-6°C for up to 42 days.
2.37.1.
3.1. 70 mL Citrate Phosphate Dextrose (CPD) anticoagulant USP for collection of 500 mL Whole Blood. Each 70 mL contains 1.79 g Dextrose (monohydrate) USP, 1.84 g Sodium Citrate (dihydrate) USP, 209 mg Citric Acid (anhydrous) USP, 156 mg Monobasic Sodium Phosphate (monohydrate) USP.
3.2. 111 mL OPTISOL Red Cell Preservative Solution. Each 111 mL contains 974 mg Sodium Chloride USP, 1.00g Dextrose (monohydrate) USP, 583 mg Mannitol USP, 33.3 mg Adenine USP.
11.1. This blood bag system includes a 16 gauge × 1 1/2 inch (1.60 × 38 mm) needle with needle cover and a 500mL (nominal capacity 600mL) collection bag containing 70mL Citrate Phosphate Dextrose (CPD) anticoagulant. The Quadruple blood bag set has one integrally attached empty primary bag, one empty satellite bag, and one satellite bag containing 111 mL OPTISOL Red Cell Preservative Solution.
11.2. Blood bag codes ending in A2 are the collection set with an Integral
11.4. The blood bag collection set is made of PVC (polyvinyl chloride with DEHP plasticizer).
11.5. The blood bag has no components made of natural rubber latex.
11.6. Tubing internal diameter (ID) nominal 3.0 mm.
11.7. Tubing outer diameter (OD) nominal 4.4 mm.
11.8. Tubing line maximum 16 segments available.
16.1. Single use only.
16.2. Sterile and non-pyrogenic fluid path. Sterilized by steam. Opacity of the blood bag system may be observed. This is due to moisture absorption during the sterilization process. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
16.3. A Material Safety Data Sheet (MSDS) is not required for this product.