Indium Dtpa In 111
(Indium In-111 Pentetate Disodium)Indium Dtpa In 111 Prescribing Information
Pentetate Indium Disodium In 111 is recommended for use in radionuclide cisternography.
Extreme care must be exercised to assure aseptic conditions in intrathecal injections.
The maximum recommended intrathecal dose in the average patient (70 kg) is 18.5 MBq, 500
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
None known.
Aseptic meningitis and pyrogenic reactions have been rarely (less than 0.4%) observed following cisternography with Pentetate lndium Disodium In 111.
One death has been reported to have occurred within 20 minutes following the administration of Pentetate Indium Disodium In 111 and appears to be drug related. In addition, two cases of septic meningitis have also been reported. There have also been reports of skin reactions and vomiting following administration of Pentetate lndium Disodium In 111. Relationship of the drug to these latter occurrences has not been established.
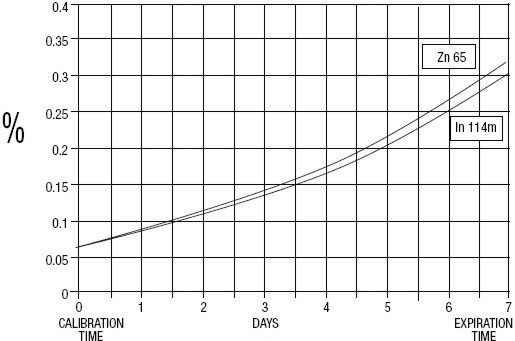
GE Healthcare (Medi-Physics, Inc.) Indium DTPA In 111 is a diagnostic drug for intrathecal use. It is available as a sterile, pyrogen-free, isotonic, aqueous solution, buffered to pH 7 to 8. At calibration time, each milliliter contains 37 MBq, 1 mCi of Pentetate Indium Disodium In 111 (no-carrier-added), 20 to 50
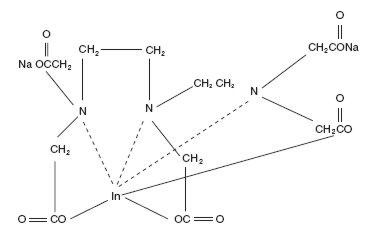
The chemical names are 1. Indate(2-)-111
Molecular formula: C14H18O10N3 111In Na2
Molecular weight: 545.29
Structural formula:
After intrathecal administration, the radiopharmaceutical is absorbed from the subarachnoid space as described below, and the remainder flows superiorly to the basal cisterns within 2 to 4 hours and subsequently will be apparent in the Sylvian cisterns, the interhemispheric cisterns, and over the cerebral convexities. In normal individuals, the radiopharmaceutical will have ascended to the parasagittal region within 24 hours with simultaneous partial or complete clearance of activity from the basal cisterns and Sylvian regions. In contrast to air, the radiopharmaceutical does not normally enter the cerebral ventricles.
Although the primary absorption of cerebrospinal fluid (CSF) into the blood stream occurs at the arachnoid villi, there is some evidence that a significant fraction of CSF is also absorbed across both the cerebral and spinal leptomeninges. Lesser quantities may also be absorbed across the ventricular ependyma. It is also generally held that these alternate routes of CSF absorption may assume primary importance when the major routes of the flow are pathologically obstructed. Approximately 65% of the administered dose is excreted by the kidneys within 24 hours and this increases to 85% in 72 hours.