Introvale Prescribing Information
Introvale is contraindicated in females who are known to have or develop the following conditions:
A high risk of arterial or venous thrombotic diseases. Examples include females who are known to:
Smoke, if over age 35
[see Boxed Warningand Warnings and Precautions (5.1)].Have current or history of deep vein thrombosis or pulmonary embolism
[see Warnings and Precautions (5.1)].Have cerebrovascular disease
[see Warnings and Precautions (5.1)].Have coronary artery disease
[see Warnings and Precautions (5.1)].Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation)
[see Warnings and Precautions (5.1)].Have inherited or acquired hypercoagulopathies
[see Warnings and Precautions (5.1)].Have uncontrolled hypertension or hypertension with vascular disease
[see Warnings and Precautions (5.4)].Have diabetes mellitus and are over age of 35, diabetes mellitus with hypertension or vascular disease or other end-organ damage, or diabetes mellitus of > 20 years duration
[see Warnings and Precautions (5.7)].Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches
[see Warnings and Precautions (5.8)].
Current diagnosis of, or history of breast cancer, which may be hormone sensitive
[see Warnings and Precautions (5.11)].Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis
[see Warnings and Precautions (5.2)and Use in Specific Populations (8.6)].Undiagnosed abnormal uterine bleeding
[see Warnings and Precautions (5.9)].Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations
[see Warnings and Precautions (5.3)].
A high risk of arterial or venous thrombotic diseases
Liver tumors or liver disease, acute viral hepatitis or decompensated cirrhosis
Undiagnosed abnormal uterine bleeding
Breast cancer
Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ ritonavir, with or without dasabuvir.
Stop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.
Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism.
Start levonorgestrel and ethinyl estradiol tablets no earlier than 4 weeks after delivery in females who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.
Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases
[see Contraindications (4)].
COCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (>35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke
Use of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1 shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1: Likelihood of Developing a VTE
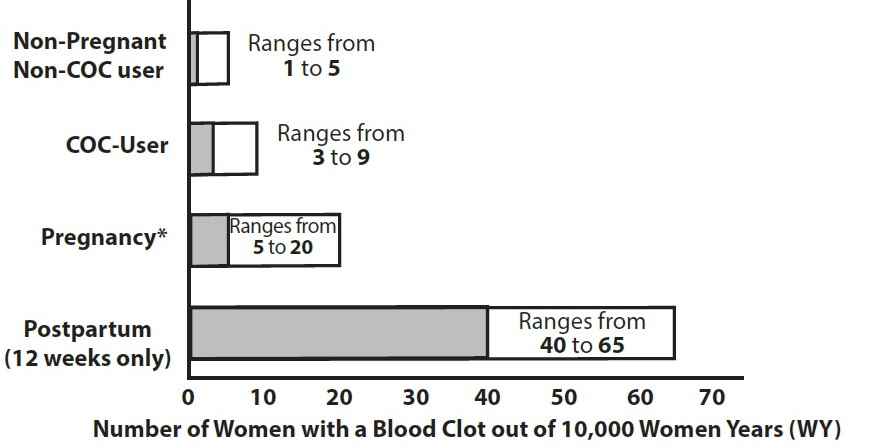
Use of levonorgestrel and ethinyl estradiol tablets provides women with more hormonal exposure on a yearly basis than conventional monthly COCs containing the same strength synthetic estrogens and progestins (an additional 9 weeks of exposure per year). In the clinical trial, one case of pulmonary embolism was reported. Postmarketing adverse reactions of VTE have been reported in women who used levonorgestrel and ethinyl estradiol tablets.

Introvale is indicated for use by females of reproductive potential to prevent pregnancy.
Take one tablet daily by mouth at the same time every day for 91 days. (
)2.1 How to Start and Take IntrovaleIntrovale is dispensed in an Extended-Cycle Blister Pack [
see How Supplied/Storage and Handling (16)]. Introvale should be started on a Sunday (see Table 1). For the first cycle of a Sunday Start regimen, an additional method of contraception should be used until after the first 7 consecutive days of administration.Table 1: Instructions for Administration of Introvale Starting Introvale in females with no current use of hormonal contraception (Sunday Start)Important:Consider the possibility of ovulation and conception prior to initiation of this product.
Tablet Color:Introvale active tablets are white to off-white (Day 1 to Day 84).
Introvale inactive tablets are green (Day 85 to Day 91).
Sunday Start:For each 91-day course, take in the following order:Take the first
white to off-whitetablet (0.15 mg of levonorgestrel and 0.03 mg ethinyl estradiol) on the first Sunday after the onset of menstruation. If menstruation begins on a Sunday, take the tablet on that day.Due to the potential risk of becoming pregnant, use additional non-hormonal contraception (such as condoms or spermicide) for the first 7 days of treatment.Take subsequent white to off-white tablets once daily at the same time each day for a total of 84 days.
Take one
greentablet (inert) daily for the following 7 days and at the same time of day that active tablets were taken. A scheduled period should occur during the 7 days that the green tablets are taken.Begin the next and all subsequent 91-day courses of Introvale without interruption on the same day of the week (Sunday) on which the patient began her first dose. Follow the same schedule as the initial 91-day course: a white to off-white tablet once a day for 84 days, and a green tablet once a day for 7 days. If the patient does not immediately start her next pill pack, instruct her to protect herself from pregnancy by using a non-hormonal back-up method of contraception until she has taken a white to off-white tablet daily for 7 consecutive days.
Switching from another contraceptive method to IntrovaleStart Introvale:Another oral contraceptiveOn the day when the new pack of the previous COC would have been started
Transdermal patchOn the day when the next application would have been scheduled.
Vaginal ringOn the day when the next insertion would have been scheduled.
InjectionOn the day when the next injection would have been scheduled.
Intrauterine contraceptive (IUD)On the day of removal.
If the IUD is not removed on first day of the patient’s menstrual cycle, additional non-hormonal contraception (such as condoms or spermicide) is needed for the first seven days of the first 91-day course.
ImplantOn the day of removal.
Starting Introvale after Abortion or MiscarriageFirst-trimesterAfter a first-trimester abortion or miscarriage, Introvale may be started immediately. An additional method of contraception is not needed if Introvale is started immediately.
If Introvale is not started within 5 days after termination of the pregnancy, the patient should use additional non-hormonal contraception (such as condoms or spermicide) for the first seven days of her first 91-day course of Introvale.
Second-trimesterDo not start Introvale until 4 weeks after a second-trimester abortion or miscarriage, due to the increased risk of thromboembolic disease. Start Introvale following the instructions in Table 1 for Sunday start. Use additional non-hormonal contraception (such as condoms or spermicide) for the first seven days of the patient’s first 91-day course of Introvale
[see Contraindications (4), Warnings and Precautions (5.1)].
Starting Introvale after ChildbirthDo not start Introvale until 4 weeks after delivery, due to the increased risk of thromboembolic disease. Start contraceptive therapy with Introvale following the instructions in Table 1 for women not currently using hormonal contraception.
Introvale is not recommended for use in lactating women
[see Use in Specific Populations (8.2)].If the woman has not yet had a period postpartum, consider the possibility of ovulation and conception occurring prior to use of Introvale
[see Contraindications (4), Warnings and Precautions (5.1), Use in Specific Populations (8.1and 8.2)].
Take tablets in the order directed on the Extended-Cycle Blister Pack. (
)2.2 Dosing IntrovaleInstruct patients to take one tablet by mouth at the same time every day. The dosing of Introvale is one white to off-white pill containing levonorgestrel and ethinyl estradiol daily for 84 consecutive days, followed by one green pill (inactive pills without hormone) for 7 days. To achieve maximum contraceptive effectiveness, Introvale must be taken exactly as directed, in the order directed on the Blister Pack, and at intervals not exceeding 24 hours. Start taking the first white to off-white pill from a new Blister Pack the very next day after taking the last green inactive pill in the Blister Pack. The failure rate may increase when pills are missed or taken incorrectly.
Introvale is available as round, biconvex, unscored tablets, packaged in a carton of 3 pouches, each pouch contains Extended-Cycle Tablet Blister Pack of 91 tablets, each containing a 13-week supply of tablets in the following order:
84 white to off-white tablets, each containing 0.15 mg of levonorgestrel and 0.03 mg ethinyl estradiol; debossed with
212on the one side and plain on the other side7 green inert tablets debossed with
279on the one side and plain on the other side.
Pregnancy: Discontinue if pregnancy occurs. (
)8.1 PregnancyRisk SummaryThere is no use for contraception in pregnancy; therefore, levonorgestrel and ethinyl estradiol tablets should be discontinued during pregnancy. Epidemiologic studies and meta-analyses have not found an increased risk of genital or non-genital birth defects (including cardiac anomalies and limb-reduction defects) following exposure to COCs before conception or during early pregnancy.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4 percent and 15 to 20 percent, respectively.
Lactation: Advise use of another contraceptive method; levonorgestrel and ethinyl estradiol tablets; may decrease milk production. (
)8.2 LactationRisk SummaryContraceptive hormones and/or metabolites are present in human milk. COCs can reduce milk production in breastfeeding females. This reduction can occur at any time but is less likely to occur once breastfeeding is well-established. When possible, advise the nursing female to use other methods of contraception until she discontinues breastfeeding
[see Dosage and Administration (2.1)]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for levonorgestrel and ethinyl estradiol tablets and any potential adverse effects on the breastfed child from levonorgestrel and ethinyl estradiol tablets or the underlying maternal condition.
Introvale is contraindicated in females who are known to have or develop the following conditions:
A high risk of arterial or venous thrombotic diseases. Examples include females who are known to:
Smoke, if over age 35
[see.andWARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTSCigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs, including Introvale, are contraindicated in women who are over 35 years of age and smoke[see Contraindications (4)and Warnings and Precautions (5.1)].WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
See full prescribing information for complete boxed warning.- Introvale is contraindicated in women over 35 years old who smoke.
- Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use.
]5.1 Thromboembolic Disorders and Other Vascular ConditionsStop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.
Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism.
Start levonorgestrel and ethinyl estradiol tablets no earlier than 4 weeks after delivery in females who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.
Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases
[see Contraindications (4)].
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (>35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke
[see Contraindications (4)]. Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs
[see Contraindications (4)]. While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman years.The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1 shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1: Likelihood of Developing a VTE

Use of levonorgestrel and ethinyl estradiol tablets provides women with more hormonal exposure on a yearly basis than conventional monthly COCs containing the same strength synthetic estrogens and progestins (an additional 9 weeks of exposure per year). In the clinical trial, one case of pulmonary embolism was reported. Postmarketing adverse reactions of VTE have been reported in women who used levonorgestrel and ethinyl estradiol tablets.

Figure 1: Likelihood of Developing a VTE Have current or history of deep vein thrombosis or pulmonary embolism
[see.]5.1 Thromboembolic Disorders and Other Vascular ConditionsStop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.
Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism.
Start levonorgestrel and ethinyl estradiol tablets no earlier than 4 weeks after delivery in females who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.
Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases
[see Contraindications (4)].
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (>35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke
[see Contraindications (4)]. Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs
[see Contraindications (4)]. While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman years.The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1 shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1: Likelihood of Developing a VTE

Use of levonorgestrel and ethinyl estradiol tablets provides women with more hormonal exposure on a yearly basis than conventional monthly COCs containing the same strength synthetic estrogens and progestins (an additional 9 weeks of exposure per year). In the clinical trial, one case of pulmonary embolism was reported. Postmarketing adverse reactions of VTE have been reported in women who used levonorgestrel and ethinyl estradiol tablets.

Figure 1: Likelihood of Developing a VTE Have cerebrovascular disease
[see.]5.1 Thromboembolic Disorders and Other Vascular ConditionsStop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.
Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism.
Start levonorgestrel and ethinyl estradiol tablets no earlier than 4 weeks after delivery in females who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.
Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases
[see Contraindications (4)].
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (>35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke
[see Contraindications (4)]. Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs
[see Contraindications (4)]. While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman years.The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1 shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1: Likelihood of Developing a VTE

Use of levonorgestrel and ethinyl estradiol tablets provides women with more hormonal exposure on a yearly basis than conventional monthly COCs containing the same strength synthetic estrogens and progestins (an additional 9 weeks of exposure per year). In the clinical trial, one case of pulmonary embolism was reported. Postmarketing adverse reactions of VTE have been reported in women who used levonorgestrel and ethinyl estradiol tablets.

Figure 1: Likelihood of Developing a VTE Have coronary artery disease
[see.]5.1 Thromboembolic Disorders and Other Vascular ConditionsStop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.
Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism.
Start levonorgestrel and ethinyl estradiol tablets no earlier than 4 weeks after delivery in females who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.
Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases
[see Contraindications (4)].
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (>35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke
[see Contraindications (4)]. Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs
[see Contraindications (4)]. While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman years.The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1 shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1: Likelihood of Developing a VTE

Use of levonorgestrel and ethinyl estradiol tablets provides women with more hormonal exposure on a yearly basis than conventional monthly COCs containing the same strength synthetic estrogens and progestins (an additional 9 weeks of exposure per year). In the clinical trial, one case of pulmonary embolism was reported. Postmarketing adverse reactions of VTE have been reported in women who used levonorgestrel and ethinyl estradiol tablets.

Figure 1: Likelihood of Developing a VTE Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation)
[see.]5.1 Thromboembolic Disorders and Other Vascular ConditionsStop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.
Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism.
Start levonorgestrel and ethinyl estradiol tablets no earlier than 4 weeks after delivery in females who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.
Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases
[see Contraindications (4)].
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (>35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke
[see Contraindications (4)]. Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs
[see Contraindications (4)]. While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman years.The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1 shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1: Likelihood of Developing a VTE

Use of levonorgestrel and ethinyl estradiol tablets provides women with more hormonal exposure on a yearly basis than conventional monthly COCs containing the same strength synthetic estrogens and progestins (an additional 9 weeks of exposure per year). In the clinical trial, one case of pulmonary embolism was reported. Postmarketing adverse reactions of VTE have been reported in women who used levonorgestrel and ethinyl estradiol tablets.

Figure 1: Likelihood of Developing a VTE Have inherited or acquired hypercoagulopathies
[see.]5.1 Thromboembolic Disorders and Other Vascular ConditionsStop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.
Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism.
Start levonorgestrel and ethinyl estradiol tablets no earlier than 4 weeks after delivery in females who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.
Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases
[see Contraindications (4)].
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (>35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke
[see Contraindications (4)]. Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs
[see Contraindications (4)]. While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman years.The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1 shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1: Likelihood of Developing a VTE

Use of levonorgestrel and ethinyl estradiol tablets provides women with more hormonal exposure on a yearly basis than conventional monthly COCs containing the same strength synthetic estrogens and progestins (an additional 9 weeks of exposure per year). In the clinical trial, one case of pulmonary embolism was reported. Postmarketing adverse reactions of VTE have been reported in women who used levonorgestrel and ethinyl estradiol tablets.

Figure 1: Likelihood of Developing a VTE Have uncontrolled hypertension or hypertension with vascular disease
[see.]5.4 HypertensionLevonorgestrel and ethinyl estradiol tablets are contraindicated in females with uncontrolled hypertension or hypertension with vascular disease
[see Contraindications (4)].For all women, including those with well-controlled hypertension, monitor blood pressure at routine visits and stop levonorgestrel and ethinyl estradiol tablets if blood pressure rises significantly.An increase in blood pressure has been reported in females taking COCs, and this increase is more likely in older women and with extended duration of use. The effect of COCs on blood pressure may vary according to the progestin in the COC.
Have diabetes mellitus and are over age of 35, diabetes mellitus with hypertension or vascular disease or other end-organ damage, or diabetes mellitus of > 20 years duration
[see.]5.7 Adverse Carbohydrate and Lipid Metabolic EffectsHyperglycemiaLevonorgestrel and ethinyl estradiol tablets are contraindicated in diabetic women over age 35, or females who have diabetes with hypertension, nephropathy, retinopathy, neuropathy, other vascular disease or females with diabetes of > 20 years of duration
[see Contraindications (4)]. Levonorgestrel and ethinyl estradiol tablets may decrease glucose tolerance. Carefully monitor prediabetic and diabetic females who are using levonorgestrel and ethinyl estradiol tablets.DyslipidemiaConsider alternative contraception for females with uncontrolled dyslipidemia. Levonorgestrel and ethinyl estradiol tablets may cause adverse lipid changes.
Females with hypertriglyceridemia, or a family history thereof, may have an increase in serum triglyceride concentrations when using levonorgestrel and ethinyl estradiol tablets, which may increase the risk of pancreatitis.
Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches
[see.]5.8 HeadacheLevonorgestrel and ethinyl estradiol tablets are contraindicated in females who have headaches with focal neurological symptoms or have migraine headaches with aura, and in women over age 35 years who have migraine headaches with or without aura
[see Contraindications (4)].If a female taking levonorgestrel and ethinyl estradiol tablets develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue levonorgestrel and ethinyl estradiol tablets if indicated. Consider discontinuation of levonorgestrel and ethinyl estradiol tablets if there is an increased frequency or severity of migraine during COC use (which may be prodromal of a cerebrovascular event)
[see Contraindications (4)].
Current diagnosis of, or history of breast cancer, which may be hormone sensitive
[see].5.11 Malignant NeoplasmsBreast CancerLevonorgestrel and ethinyl estradiol tablets are contraindicated in females who currently have or have had breast cancer because breast cancer may be hormonally sensitive
[see Contraindications (4)].Epidemiology studies have not found a consistent association between use of combined oral contraceptives (COCs) and breast cancer risk. Studies do not show an association between ever (current or past) use of COCs and risk of breast cancer. However, some studies report a small increase in the risk of breast cancer among current or recent users (<6 months since last use) and current users with longer duration of COC use
[see Postmarketing Experience (6.2)].Cervical CancerSome studies suggest that COC are associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there is controversy about the extent to which such findings may be due to differences in sexual behavior and other factors.
Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis
[see.and5.2 Liver DiseaseElevated Liver EnzymesLevonorgestrel and ethinyl estradiol tablets are contraindicated in females with acute viral hepatitis or severe (decompensated) cirrhosis of the liver
[see Contraindications (4)]. Acute liver test abnormalities may necessitate the discontinuation of levonorgestrel and ethinyl estradiol tablets until the liver tests return to normal and levonorgestrel and ethinyl estradiol tablets causation has been excluded. Discontinue levonorgestrel and ethinyl estradiol tablets if jaundice develops.Liver TumorsLevonorgestrel and ethinyl estradiol tablets are contraindicated in females with benign and malignant liver tumors
[see Contraindications (4)]. COCs increase the risk of hepatic adenomas. An estimate of the attributable risk is 3.3 cases/100,000 COC users. Rupture of hepatic adenomas may cause death through intra-abdominal hemorrhage.Studies have shown an increased risk of developing hepatocellular carcinoma in long-term (> 8 years) COC users. The attributable risk of liver cancers in COC users is less than one case per million users.
]8.6 Hepatic ImpairmentThe pharmacokinetics of levonorgestrel and ethinyl estradiol tablets have not been studied in subjects with hepatic impairment. However, COCs may be poorly metabolized in patients with hepatic impairment. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with acute hepatitis or severe decompensated cirrhosis
[see Contraindications (4)and Warnings and Precautions (5.2)].Undiagnosed abnormal uterine bleeding
[see.]5.9 Bleeding Irregularities and AmenorrheaBleeding and/or spotting that occurs at any time while taking the first 84 tablets of each extended-cycle regimen is considered “unscheduled” bleeding/spotting. Bleeding that occurs during the time a woman takes the seven green inert tablets is considered “scheduled” bleeding.
Unscheduled Bleeding and SpottingFemales using levonorgestrel and ethinyl estradiol tablets may experience unscheduled (breakthrough or intracyclic) bleeding and spotting especially during the first 3 months of use. Bleeding irregularities may resolve over time or by changing to a different contraceptive product. If unscheduled bleeding persists or occurs after previously regular cycles, evaluate for causes such as pregnancy or malignancy.
Before prescribing levonorgestrel and ethinyl estradiol tablets, advise the woman to weigh the occurrence of fewer scheduled menses (4 per year instead of 13 per year) against the occurrence of increased unscheduled bleeding and/or spotting.
The clinical trial of the efficacy of levonorgestrel and ethinyl estradiol tablets (91-day cycles) in preventing pregnancy also assessed scheduled and unscheduled bleeding. The participants in the study were composed primarily of women who had used oral contraceptives previously as opposed to new users. Women with a history of breakthrough bleeding/spotting ≥ 10 consecutive days on oral contraceptives were excluded from the study. More levonorgestrel and ethinyl estradiol tablets subjects, compared to subjects on the comparator 28-day cycle regimen, discontinued prematurely for unacceptable bleeding (7.7% [levonorgestrel and ethinyl estradiol tablets] vs. 1.8% [28-day cycle regimen]).
Unscheduled bleeding and unscheduled spotting decreased over successive 91-day cycles. Table 3 below presents the number of days with unscheduled bleeding and/or spotting for each respective 91-day cycle.
Table 3: Number of Unscheduled Bleeding and/or Spotting Days per 91-day Cycle Q1=Quartile 1: 25% of women had ≤ this number of days of unscheduled bleeding/spotting Median: 50% of women had ≤ this number of days of unscheduled bleeding/spotting Q3=Quartile 3: 75% of women had ≤ this number of days of unscheduled bleeding/spotting Cycle (N)Days of Unscheduled Bleeding and/or Spotting per 84-Day IntervalMedian Days Per Subject-MonthMeanQ1MedianQ31 (446)
15.1
3.0
12
23.0
3.0
2 (368)
11.6
2.0
6
17.5
1.5
3 (309)
10.6
1.0
6
15.0
1.5
4 (282)
8.8
1.0
4
14.0
1.0
Table 4 shows the percentages of women with ≥ 7 days and ≥ 20 days of unscheduled spotting and/or bleeding in the levonorgestrel and ethinyl estradiol tablets and the 28-day cycle treatment groups.
Table 4: Percentage of Subjects with Unscheduled Bleeding and/or Spotting Days of unscheduled bleeding and/or spotting
Percentage of Subjectsa
Levonorgestrel and Ethinyl Estradiol TabletsCycle 1 (N=385)
Cycle 4 (N=261)
≥ 7 days
65%
42%
≥ 20 days
35%
15%
28-day regimenCycles 1-4 (N=194)
Cycles 10-13 (N=158)
≥ 7 days
38%
39%
≥ 20 days
6%
4%
aBased on spotting and/or bleeding on days 1-84 of a 91 day cycle in the levonorgestrel and ethinyl estradiol tablets subjects and days 1-21 of a 28 day cycle over 4 cycles in the 28-day dosing regimen.
Total days of bleeding and/or spotting (scheduled plus unscheduled) were similar over one year of treatment for levonorgestrel and ethinyl estradiol tablets subjects and subjects on the 28-day cycle regimen.
Amenorrhea and OligomenorrheaFemales who use levonorgestrel and ethinyl estradiol tablets may experience absence of scheduled (withdrawal) bleeding, even if they are not pregnant. Based on data from the clinical trial of levonorgestrel and ethinyl estradiol tablets, amenorrhea occurred in approximately 0.8% of females during Cycle 1, 1.2% of females during Cycle 2, 3.7% of females during Cycle 3, and 3.4% of females during Cycle 4.
Because females using levonorgestrel and ethinyl estradiol tablets will likely have scheduled bleeding only 4 times per year, rule out pregnancy at the time of any missed menstrual period.
After discontinuation of levonorgestrel and ethinyl estradiol tablets, amenorrhea or oligomenorrhea may occur, especially if these conditions were pre-existent.
Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations
[see.]5.3 Risk of Liver Enzyme Elevations with Concomitant Hepatitis C TreatmentDuring clinical trials with the Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications, such as levonorgestrel and ethinyl estradiol tablets. Discontinue levonorgestrel and ethinyl estradiol tablets prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir
[see Contraindications (4)]. Levonorgestrel and ethinyl estradiol tablets can be restarted approximately 2 weeks following completion of treatment with the Hepatitis C combination drug regimen.